

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50326719 (S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tolylpiperazin-1-ylsulfonyl)methyl)bicyclo[2.2.1]heptan-2-yl)-4-(methylsulfonyl)butanamide::CHEMBL1253853
SMILES: Cc1ccccc1N1CCN(CC1)S(=O)(=O)C[C@]12CC[C@H](C[C@@H]1NC(=O)[C@@H](N)CCS(C)(=O)=O)C2(C)C
InChI Key: InChIKey=MWIASLNTAGRGGA-ZJPWWDJASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (RAT) | BDBM50326719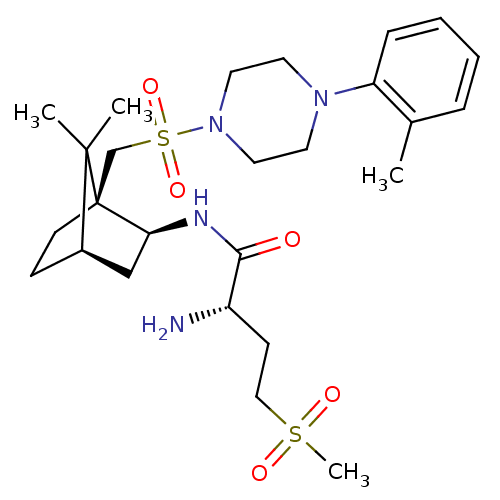 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in rat uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yerkes National Primate Research Center Curated by ChEMBL | Assay Description Binding affinity to human OT receptor | Bioorg Med Chem Lett 23: 902-6 (2013) Article DOI: 10.1016/j.bmcl.2012.10.116 BindingDB Entry DOI: 10.7270/Q29Z9690 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-oxytocin from oxytocin receptor in human uterus tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AVPR1A (RAT) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V1a receptor in rat liver tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V1a receptor in human liver tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 Receptor (Rattus norvegicus (Rat)) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V2 receptor in rat kidney tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V2 receptor in human kidney tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro antagonism of OT-induced uterine contractions,against rat uterus by measuring pA2 value which refers to the negative logarithm of the molar ... | J Med Chem 37: 565-71 (1994) BindingDB Entry DOI: 10.7270/Q21C1VZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AVPR1A (RAT) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]AVP from binding to arginine vasopressin 1a (V1a) column of rat liver | J Med Chem 37: 565-71 (1994) BindingDB Entry DOI: 10.7270/Q21C1VZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Yerkes National Primate Research Center Curated by ChEMBL | Assay Description Inhibition of rat uterus OT receptor | Bioorg Med Chem Lett 23: 902-6 (2013) Article DOI: 10.1016/j.bmcl.2012.10.116 BindingDB Entry DOI: 10.7270/Q29Z9690 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-OT from binding to oxytocin receptor of rat uterus | J Med Chem 37: 565-71 (1994) BindingDB Entry DOI: 10.7270/Q21C1VZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 Receptor (Rattus norvegicus (Rat)) | BDBM50326719 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from binding to Vasopressin receptor V2 of rat kidney | J Med Chem 37: 565-71 (1994) BindingDB Entry DOI: 10.7270/Q21C1VZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||