

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50327391 CHEMBL1258976::N1-(4-(2-chlorophenyl)-6-(2-(isopropylamino)thiazol-5-yl)pyrimidin-2-yl)-N2,N2-dimethylethane-1,2-diamine
SMILES: CC(C)Nc1ncc(s1)-c1cc(nc(NCCN(C)C)n1)-c1ccccc1Cl
InChI Key: InChIKey=WTRQXHALVSEXHZ-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50327391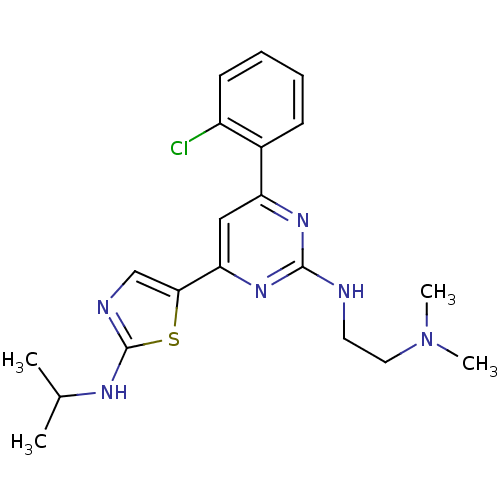 (CHEMBL1258976 | N1-(4-(2-chlorophenyl)-6-(2-(isopr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of LIMK1 | Bioorg Med Chem Lett 20: 5864-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.102 BindingDB Entry DOI: 10.7270/Q22F7NP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50327391 (CHEMBL1258976 | N1-(4-(2-chlorophenyl)-6-(2-(isopr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of bacterially expressed activated p38alpha pre-incubated 10 mins measured after 45 mins by scintillation counting | Bioorg Med Chem Lett 20: 5864-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.102 BindingDB Entry DOI: 10.7270/Q22F7NP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50327391 (CHEMBL1258976 | N1-(4-(2-chlorophenyl)-6-(2-(isopr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of LIMK2 | Bioorg Med Chem Lett 20: 5864-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.102 BindingDB Entry DOI: 10.7270/Q22F7NP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||