

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50328120 1-benzyl-N-(4-(trifluoromethoxy)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine::CHEMBL1258245::US9670214, TABLE 10.20
SMILES: FC(F)(F)Oc1ccc(Nc2ncnc3n(Cc4ccccc4)ncc23)cc1
InChI Key: InChIKey=HCPAYDMQUBMGRZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bcr-Abl (Homo sapiens (Human)) | BDBM50328120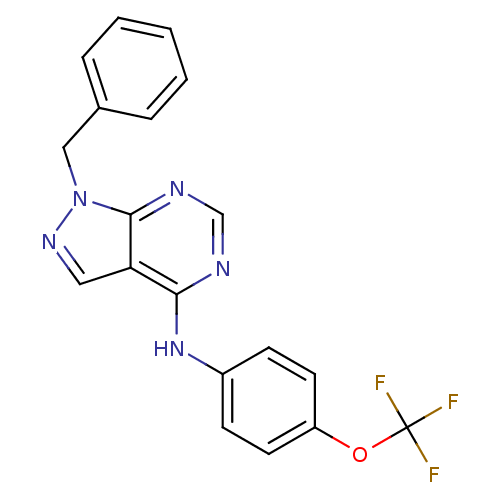 (1-benzyl-N-(4-(trifluoromethoxy)phenyl)-1H-pyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of Bcr-Abl in mouse BA/F3 cells | J Med Chem 53: 6934-46 (2010) Article DOI: 10.1021/jm100555f BindingDB Entry DOI: 10.7270/Q2DJ5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BCR/ABL fusion protein (T351I) (Homo sapiens (Human)) | BDBM50328120 (1-benzyl-N-(4-(trifluoromethoxy)phenyl)-1H-pyrazol...) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; THE SCRIPPS RESEARCH INSTITUTE US Patent | Assay Description In vitro kinase assays were carried out by using recombinant murine c-abl containing SH3, SH2 and kinase domains (residues 46-531) and full length im... | US Patent US9670214 (2017) BindingDB Entry DOI: 10.7270/Q2000086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BCR/ABL fusion protein isoform X3 (Homo sapiens (Human)) | BDBM50328120 (1-benzyl-N-(4-(trifluoromethoxy)phenyl)-1H-pyrazol...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; THE SCRIPPS RESEARCH INSTITUTE US Patent | Assay Description In vitro kinase assays were carried out by using recombinant murine c-abl containing SH3, SH2 and kinase domains (residues 46-531) and full length im... | US Patent US9670214 (2017) BindingDB Entry DOI: 10.7270/Q2000086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||