 Found 5 hits for monomerid = 50328854
Found 5 hits for monomerid = 50328854 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50328854
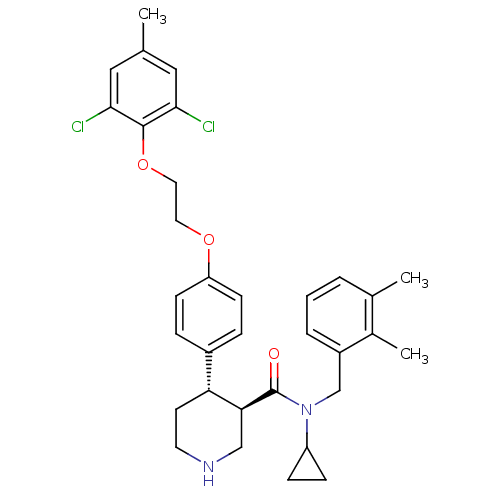
(CHEMBL1270661 | rac-N-cyclopropyl-4-(4-(2-(2,6-dic...)Show SMILES Cc1cc(Cl)c(OCCOc2ccc(cc2)[C@H]2CCNC[C@@H]2C(=O)N(Cc2cccc(C)c2C)C2CC2)c(Cl)c1 |r| Show InChI InChI=1S/C33H38Cl2N2O3/c1-21-17-30(34)32(31(35)18-21)40-16-15-39-27-11-7-24(8-12-27)28-13-14-36-19-29(28)33(38)37(26-9-10-26)20-25-6-4-5-22(2)23(25)3/h4-8,11-12,17-18,26,28-29,36H,9-10,13-16,19-20H2,1-3H3/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using testosterone as substrate |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50328854

(CHEMBL1270661 | rac-N-cyclopropyl-4-(4-(2-(2,6-dic...)Show SMILES Cc1cc(Cl)c(OCCOc2ccc(cc2)[C@H]2CCNC[C@@H]2C(=O)N(Cc2cccc(C)c2C)C2CC2)c(Cl)c1 |r| Show InChI InChI=1S/C33H38Cl2N2O3/c1-21-17-30(34)32(31(35)18-21)40-16-15-39-27-11-7-24(8-12-27)28-13-14-36-19-29(28)33(38)37(26-9-10-26)20-25-6-4-5-22(2)23(25)3/h4-8,11-12,17-18,26,28-29,36H,9-10,13-16,19-20H2,1-3H3/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328854

(CHEMBL1270661 | rac-N-cyclopropyl-4-(4-(2-(2,6-dic...)Show SMILES Cc1cc(Cl)c(OCCOc2ccc(cc2)[C@H]2CCNC[C@@H]2C(=O)N(Cc2cccc(C)c2C)C2CC2)c(Cl)c1 |r| Show InChI InChI=1S/C33H38Cl2N2O3/c1-21-17-30(34)32(31(35)18-21)40-16-15-39-27-11-7-24(8-12-27)28-13-14-36-19-29(28)33(38)37(26-9-10-26)20-25-6-4-5-22(2)23(25)3/h4-8,11-12,17-18,26,28-29,36H,9-10,13-16,19-20H2,1-3H3/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in plasma |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50328854

(CHEMBL1270661 | rac-N-cyclopropyl-4-(4-(2-(2,6-dic...)Show SMILES Cc1cc(Cl)c(OCCOc2ccc(cc2)[C@H]2CCNC[C@@H]2C(=O)N(Cc2cccc(C)c2C)C2CC2)c(Cl)c1 |r| Show InChI InChI=1S/C33H38Cl2N2O3/c1-21-17-30(34)32(31(35)18-21)40-16-15-39-27-11-7-24(8-12-27)28-13-14-36-19-29(28)33(38)37(26-9-10-26)20-25-6-4-5-22(2)23(25)3/h4-8,11-12,17-18,26,28-29,36H,9-10,13-16,19-20H2,1-3H3/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using testosterone as substrate |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328854

(CHEMBL1270661 | rac-N-cyclopropyl-4-(4-(2-(2,6-dic...)Show SMILES Cc1cc(Cl)c(OCCOc2ccc(cc2)[C@H]2CCNC[C@@H]2C(=O)N(Cc2cccc(C)c2C)C2CC2)c(Cl)c1 |r| Show InChI InChI=1S/C33H38Cl2N2O3/c1-21-17-30(34)32(31(35)18-21)40-16-15-39-27-11-7-24(8-12-27)28-13-14-36-19-29(28)33(38)37(26-9-10-26)20-25-6-4-5-22(2)23(25)3/h4-8,11-12,17-18,26,28-29,36H,9-10,13-16,19-20H2,1-3H3/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data