

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50328952 CHEMBL1269766::N,N'-(Iminodiheptane-7,1-diyl)bis[5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide]
SMILES: Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NCCCCCCCNCCCCCCCNC(=O)c1nn(c(c1C)-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl
InChI Key: InChIKey=PLKCOAWMCVGORE-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50328952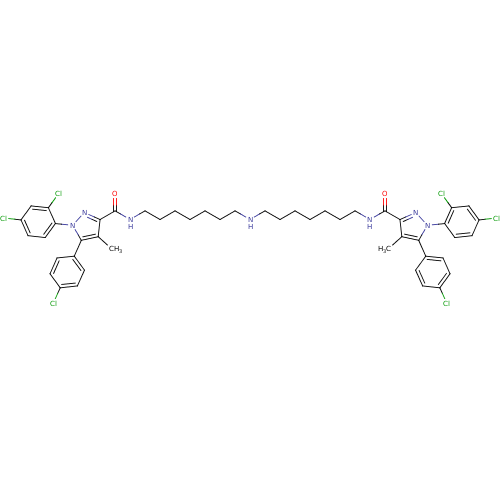 (CHEMBL1269766 | N,N'-(Iminodiheptane-7,1-diyl)bis[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 53: 7048-60 (2010) Article DOI: 10.1021/jm1006676 BindingDB Entry DOI: 10.7270/Q27P8ZMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50328952 (CHEMBL1269766 | N,N'-(Iminodiheptane-7,1-diyl)bis[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 53: 7048-60 (2010) Article DOI: 10.1021/jm1006676 BindingDB Entry DOI: 10.7270/Q27P8ZMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50328952 (CHEMBL1269766 | N,N'-(Iminodiheptane-7,1-diyl)bis[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 553 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in CHOK1 cells | J Med Chem 53: 7048-60 (2010) Article DOI: 10.1021/jm1006676 BindingDB Entry DOI: 10.7270/Q27P8ZMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50328952 (CHEMBL1269766 | N,N'-(Iminodiheptane-7,1-diyl)bis[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 179 | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Antagonist activity at human brain CB1 receptor assessed as inhibition for [35S]GTPgammaS binding | J Med Chem 53: 7048-60 (2010) Article DOI: 10.1021/jm1006676 BindingDB Entry DOI: 10.7270/Q27P8ZMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50328952 (CHEMBL1269766 | N,N'-(Iminodiheptane-7,1-diyl)bis[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 871 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50328952 (CHEMBL1269766 | N,N'-(Iminodiheptane-7,1-diyl)bis[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 871 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Inverse agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Gbeta1gamma2 and RSG4 assessed as degradation of [gamma-33P]GTP af... | Bioorg Med Chem Lett 24: 4209-14 (2014) Article DOI: 10.1016/j.bmcl.2014.07.038 BindingDB Entry DOI: 10.7270/Q26M38HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50328952 (CHEMBL1269766 | N,N'-(Iminodiheptane-7,1-diyl)bis[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Antagonist activity at rat brain CB1 receptor assessed as inhibition of [35S]GTPgammaS binding | J Med Chem 53: 7048-60 (2010) Article DOI: 10.1021/jm1006676 BindingDB Entry DOI: 10.7270/Q27P8ZMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||