

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50330793 2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one::CHEMBL1275969
SMILES: CC(C)c1ccccc(=O)c1O
InChI Key: InChIKey=TUFYVOCKVJOUIR-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50330793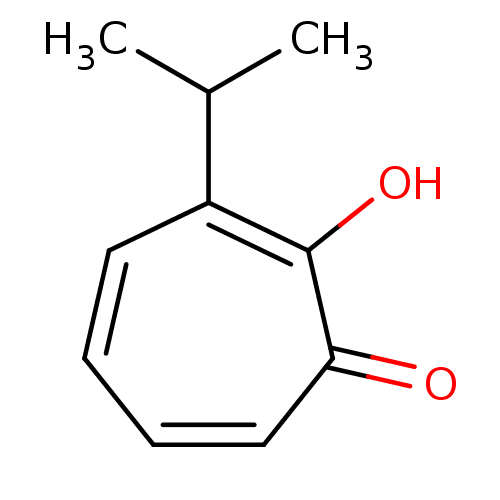 (2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase after 15 mins by Lineweaver-Bulk plot analysis | Bioorg Med Chem 18: 8112-8 (2010) Article DOI: 10.1016/j.bmc.2010.08.056 BindingDB Entry DOI: 10.7270/Q2JH3MF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50330793 (2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase | Bioorg Med Chem 22: 6193-200 (2014) Article DOI: 10.1016/j.bmc.2014.08.027 BindingDB Entry DOI: 10.7270/Q2DV1MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50330793 (2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50330793 (2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50330793 (2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one ...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Competitive inhibition of tyrosinase in human G-361 cells incubated for 10 mins measured for 2 hrs by MBTH-based spectrophotometry | Bioorg Med Chem 22: 6193-200 (2014) Article DOI: 10.1016/j.bmc.2014.08.027 BindingDB Entry DOI: 10.7270/Q2DV1MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50330793 (2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Theoretical Medicine, Inc. Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 15 mins | Bioorg Med Chem 18: 8112-8 (2010) Article DOI: 10.1016/j.bmc.2010.08.056 BindingDB Entry DOI: 10.7270/Q2JH3MF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||