

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50331042 2-(2-(((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,10-dioxo-1,2-dithia-5,9-diazacyclotridecane-4-carboxamido)methyl)phenyl)acetic acid::CHEMBL1277623
SMILES: N[C@H]1CCSSC[C@H](NC(=O)C[C@H](Cc2ccc(O)cc2)NC1=O)C(=O)NCc1ccccc1CC(O)=O
InChI Key: InChIKey=VZYRMNZEFCZSER-BVSLBCMMSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331042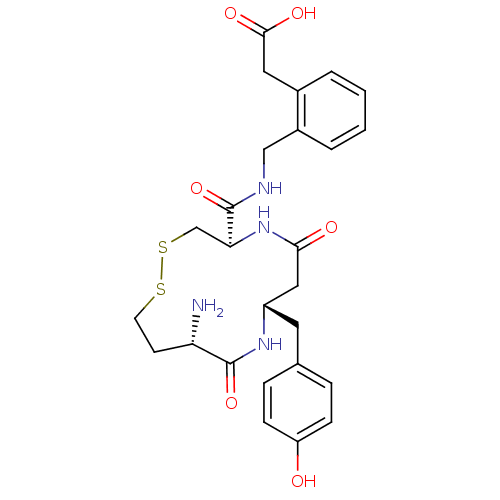 (2-(2-(((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human recombinant IRAP expressed in HEK293 cells | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor antagonist protein (Homo sapiens) | BDBM50331042 (2-(2-(((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331042 (2-(2-(((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]AL-11 from human IRAP expressed in CHO-K1 cells after 30 mins by liquid scintillation counting in presence of 30 mM EDTA/600 uM 1... | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM50331042 (2-(2-(((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]AL-11 from human IRAP expressed in CHO-K1 cells after 30 mins by liquid scintillation counting in absence of 30 mM EDTA/600 uM 1,... | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50331042 (2-(2-(((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 7.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human recombinant aminopeptidase N expressed in HEK293 cells | J Med Chem 53: 8059-71 (2010) Article DOI: 10.1021/jm100793t BindingDB Entry DOI: 10.7270/Q23B60C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor antagonist protein (Homo sapiens) | BDBM50331042 (2-(2-(((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase (Homo sapiens (Human)) | BDBM50331042 (2-(2-(((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Scientific Research Demokritos Curated by ChEMBL | Assay Description Inhibition of ERAP2 (unknown origin) using Arg-AMC as substrate by fluorescence based assay | ACS Med Chem Lett 11: 1429-1434 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor antagonist protein (Homo sapiens) | BDBM50331042 (2-(2-(((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Scientific Research Demokritos Curated by ChEMBL | Assay Description Inhibition of human IRAP soluble domain expressed in HEK293S GnTI(-) cells using Leu-AMC as substrate incubated for 2 hrs by fluorescence based assay | ACS Med Chem Lett 11: 1429-1434 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor antagonist protein (Homo sapiens) | BDBM50331042 (2-(2-(((4R,8S,11S)-11-amino-8-(4-hydroxybenzyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Scientific Research Demokritos Curated by ChEMBL | Assay Description Inhibition of human IRAP soluble domain expressed in HEK293S GnTI(-) cells using Arg-AMC as substrate incubated for 2 hrs by fluorescence based assay | ACS Med Chem Lett 11: 1429-1434 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||