

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50333930 CHEMBL1644419::[(2R,6R,10S,15S,17R)-13-benzyl-6-hydroxy-4,17-dimethyl-5-oxo-15-(prop-1-en-2-yl)-12,14,18-trioxapentacyclo[11.4.1.0^{1,10}.0^{2,6}.0^{11,15}]octadeca-3,8-dien-8-yl]methyl 2-(4-amino-3-methoxyphenyl)acetate
SMILES: COc1cc(CC(=O)OCC2=C[C@H]3C4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)C3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1N
InChI Key: InChIKey=SNPANJIXYQFRNW-XSOHGINVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vanilloid receptor (Rattus norvegicus (rat)) | BDBM50333930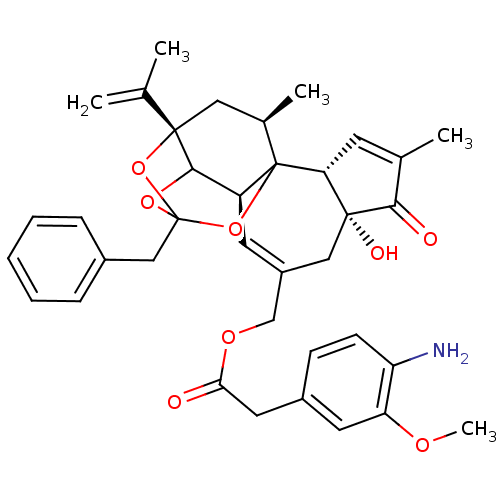 (CHEMBL1644419 | [(2R,6R,10S,15S,17R)-13-benzyl-6-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from rat TRV1 expressed in CHO cells by competitive binding assay | Bioorg Med Chem Lett 21: 299-302 (2010) Article DOI: 10.1016/j.bmcl.2010.11.012 BindingDB Entry DOI: 10.7270/Q2J967BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vanilloid receptor (Rattus norvegicus (rat)) | BDBM50333930 (CHEMBL1644419 | [(2R,6R,10S,15S,17R)-13-benzyl-6-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at rat TRPV1 expressed in CHO cells assessed as 45Ca2+ uptake | Bioorg Med Chem Lett 21: 299-302 (2010) Article DOI: 10.1016/j.bmcl.2010.11.012 BindingDB Entry DOI: 10.7270/Q2J967BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||