

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50334346 4-(3-(2-cyanophenyl)ureido)benzenesulfonamide::4-{([(2'-Cyanophenyl)amino]carbonyl)amino}benzenesulfonamide::CA IX inhibitor, (C)3::CHEMBL1643301
SMILES: NS(=O)(=O)c1ccc(NC(=O)Nc2ccccc2C#N)cc1
InChI Key: InChIKey=HOUIRAFTHIMUSN-UHFFFAOYSA-N
Data: 8 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50334346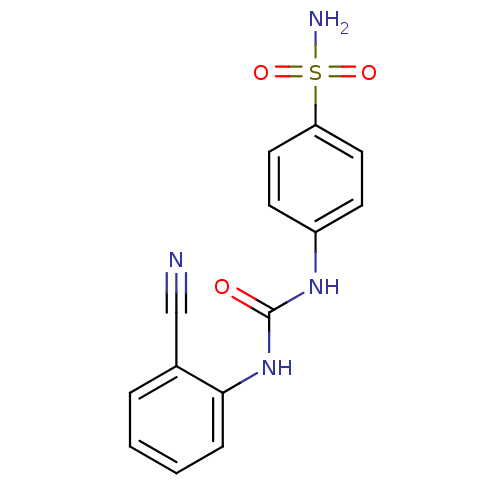 (4-(3-(2-cyanophenyl)ureido)benzenesulfonamide | 4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency | Assay Description The inhibition constants (Ki) of FEC to four human CA isoenzymes I, II, IX and XII were determined by CA catalyzed CO2 hydration assays following pre... | J Enzyme Inhib Med Chem 29: 249-55 (2014) Article DOI: 10.3109/14756366.2013.773994 BindingDB Entry DOI: 10.7270/Q2VM4B6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50334346 (4-(3-(2-cyanophenyl)ureido)benzenesulfonamide | 4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of tumor-associated human carbonic anhydrase 9 preincubated for 15 min by CO2 hydration assay | J Med Chem 54: 1896-902 (2011) Article DOI: 10.1021/jm101541x BindingDB Entry DOI: 10.7270/Q2CC110M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50334346 (4-(3-(2-cyanophenyl)ureido)benzenesulfonamide | 4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem Lett 21: 102-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.064 BindingDB Entry DOI: 10.7270/Q2WS8TH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50334346 (4-(3-(2-cyanophenyl)ureido)benzenesulfonamide | 4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 2 preincubated for 15 min by CO2 hydration assay | J Med Chem 54: 1896-902 (2011) Article DOI: 10.1021/jm101541x BindingDB Entry DOI: 10.7270/Q2CC110M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50334346 (4-(3-(2-cyanophenyl)ureido)benzenesulfonamide | 4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of tumor-associated human carbonic anhydrase 12 preincubated for 15 min by CO2 hydration assay | J Med Chem 54: 1896-902 (2011) Article DOI: 10.1021/jm101541x BindingDB Entry DOI: 10.7270/Q2CC110M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50334346 (4-(3-(2-cyanophenyl)ureido)benzenesulfonamide | 4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human cytosolic carbonic anhydrase 1 preincubated for 15 min by CO2 hydration assay | J Med Chem 54: 1896-902 (2011) Article DOI: 10.1021/jm101541x BindingDB Entry DOI: 10.7270/Q2CC110M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PROBABLE TRANSMEMBRANE CARBONIC ANHYDRASE (CARBONATE DEHYDRATASE) (CARBONIC DEHYDRATASE) (Mycobacterium tuberculosis) | BDBM50334346 (4-(3-(2-cyanophenyl)ureido)benzenesulfonamide | 4-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 463 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant beta-carbonic anhydrase Rv3273 preincubated for 15 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem Lett 21: 102-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.064 BindingDB Entry DOI: 10.7270/Q2WS8TH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase (mtCA 1) (Mycobacterium tuberculosis) | BDBM50334346 (4-(3-(2-cyanophenyl)ureido)benzenesulfonamide | 4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 534 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant beta-carbonic anhydrase Rv1284 preincubated for 15 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem Lett 21: 102-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.064 BindingDB Entry DOI: 10.7270/Q2WS8TH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||