

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50335394 CHEMBL1651626::N-Octyl-L-ido-1-deoxynojirimycin
SMILES: CCCCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@@H]1CO
InChI Key: InChIKey=VGSQNAQYXKTCLP-IGQOVBAYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335394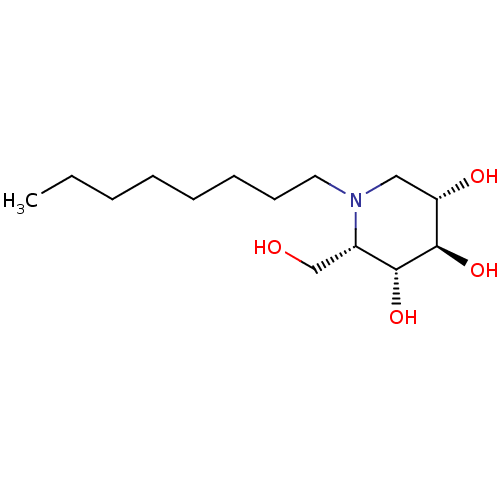 (CHEMBL1651626 | N-Octyl-L-ido-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335394 (CHEMBL1651626 | N-Octyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Homo sapiens (Human)) | BDBM50335394 (CHEMBL1651626 | N-Octyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335394 (CHEMBL1651626 | N-Octyl-L-ido-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335394 (CHEMBL1651626 | N-Octyl-L-ido-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| alpha-glucosidase (Gaa) (Mus musculus (Mouse)) | BDBM50335394 (CHEMBL1651626 | N-Octyl-L-ido-1-deoxynojirimycin) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||