

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50335508 1-(4-(6-benzylbenzo[d]thiazol-2-yl)-3-fluorobenzyl)azetidine-3-carboxylic acid::CHEMBL1651854
SMILES: OC(=O)C1CN(Cc2ccc(-c3nc4ccc(Cc5ccccc5)cc4s3)c(F)c2)C1
InChI Key: InChIKey=YHUJYSUWHIWQKN-UHFFFAOYSA-N
Data: 4 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50335508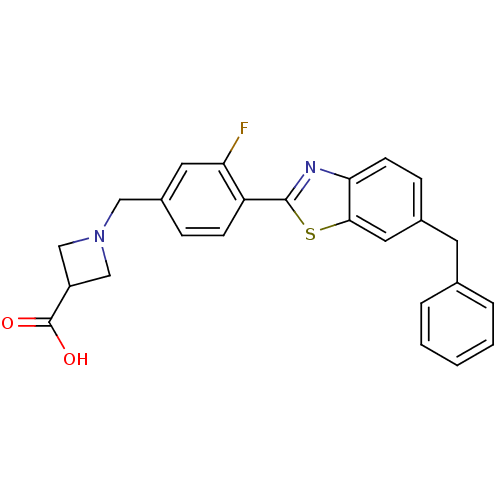 (1-(4-(6-benzylbenzo[d]thiazol-2-yl)-3-fluorobenzyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3R expressed in CHO cells by Ca(2+) mobilization assay | ACS Med Chem Lett 2: 107-112 (2011) Article DOI: 10.1021/ml100306h BindingDB Entry DOI: 10.7270/Q2H995G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50335508 (1-(4-(6-benzylbenzo[d]thiazol-2-yl)-3-fluorobenzyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 221 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P1 receptor expressed in human U2OS cells co-expressing eGFP assessed as receptor internalization after 1 hr | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50335508 (1-(4-(6-benzylbenzo[d]thiazol-2-yl)-3-fluorobenzyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells co-expressing Gq/i5 G-protein assessed as calcium mobilization by FLIPR assay | ACS Med Chem Lett 2: 102-106 (2011) Article DOI: 10.1021/ml100228m BindingDB Entry DOI: 10.7270/Q2T72HR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50335508 (1-(4-(6-benzylbenzo[d]thiazol-2-yl)-3-fluorobenzyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 221 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Induction of human GFP-tagged chimeric S1P1R internalization in human U2Os cells by fluorescence microscopy | ACS Med Chem Lett 2: 107-112 (2011) Article DOI: 10.1021/ml100306h BindingDB Entry DOI: 10.7270/Q2H995G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||