

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50335522 (2S,4R)-N-((1R,2R)-2-hydroxy-1-((2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(methylthio)-tetrahydro-2H-pyran-2-yl)propyl)-1-methyl-4-propylpyrrolidine-2-carboxamide::(2S,4R)-N-((1S)-2-hydroxy-1-((2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(methylthio)-tetrahydro-2H-pyran-2-yl)propyl)-1-methyl-4-propylpyrrolidine-2-carboxamide::1-Methyl-4-propyl-pyrrolidine-2-carboxylic acid [2-hydroxy-1-(3,4,5-trihydroxy-6-methylsulfanyl-tetrahydro-pyran-2-yl)-propyl]-amide::CHEMBL1447::Cillimycin::Frademicina::LINCOMYCIN::Lincocin::Lincomycin hydrochloride::lincomycin A
SMILES: CCC[C@@H]1C[C@H](N(C)C1)C(=O)N[C@H]([C@@H](C)O)[C@H]1O[C@H](SC)[C@H](O)[C@@H](O)[C@H]1O
InChI Key: InChIKey=OJMMVQQUTAEWLP-KIDUDLJLSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50335522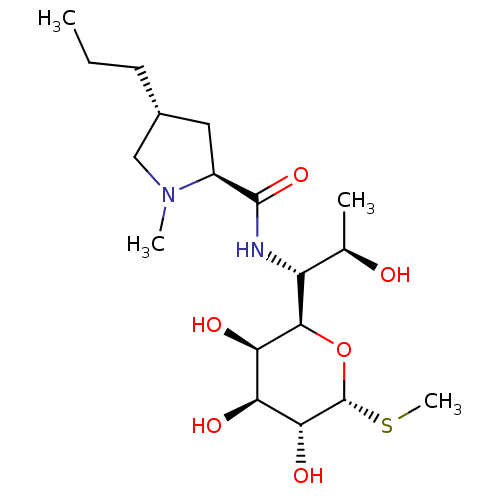 ((2S,4R)-N-((1R,2R)-2-hydroxy-1-((2R,3R,4S,5R,6R)-3...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.83E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Atatürk University | Assay Description PON activities were measured in the presence of different drug concentrations. Control activity was assumed to be 100% in the absence of inhibitor. E... | J Enzyme Inhib Med Chem 28: 758-64 (2013) Article DOI: 10.3109/14756366.2012.681653 BindingDB Entry DOI: 10.7270/Q25B01CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum albumin (Homo sapiens (Human)) | BDBM50335522 ((2S,4R)-N-((1R,2R)-2-hydroxy-1-((2R,3R,4S,5R,6R)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a |
CNRS-CPBS/UMR 5236 Curated by ChEMBL | Assay Description Binding affinity to first site on human serum albumin by SPR | Antimicrob Agents Chemother 53: 1528-31 (2009) Article DOI: 10.1128/AAC.00971-08 BindingDB Entry DOI: 10.7270/Q2SN09ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||