 Found 13 hits for monomerid = 50335523
Found 13 hits for monomerid = 50335523 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Solute carrier family 22 member 8
(Homo sapiens (Human)) | BDBM50335523
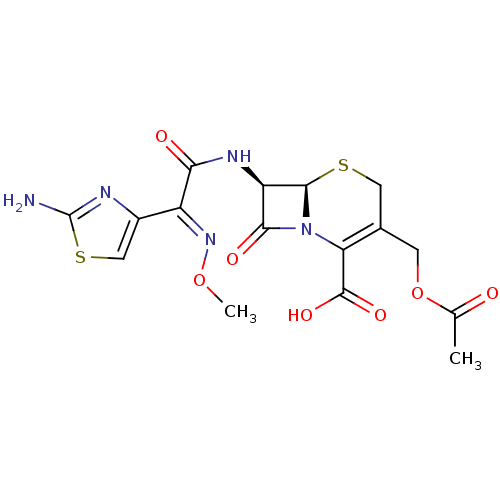
((6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-y...)Show SMILES CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16| Show InChI InChI=1S/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/b20-9+/t10-,14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of Estrone sulfate uptake in OAT3-expressing S2 cells |
Eur J Pharmacol 438: 137-42 (2002)
BindingDB Entry DOI: 10.7270/Q2N87C2C |
More data for this
Ligand-Target Pair | |
Beta-lactamase ADC-33
(Acinetobacter baumannii) | BDBM50335523

((6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-y...)Show SMILES CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16| Show InChI InChI=1S/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/b20-9+/t10-,14-/m1/s1 | PDB
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Activity of Acinetobacter baumannii ADC-33 beta-lactamase |
Antimicrob Agents Chemother 54: 3484-8 (2010)
Article DOI: 10.1128/AAC.00050-10
BindingDB Entry DOI: 10.7270/Q2348KM2 |
More data for this
Ligand-Target Pair | |
ADC-11
(Acinetobacter baumannii) | BDBM50335523

((6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-y...)Show SMILES CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16| Show InChI InChI=1S/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/b20-9+/t10-,14-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Activity of Acinetobacter baumannii ADC-11 beta-lactamase |
Antimicrob Agents Chemother 54: 3484-8 (2010)
Article DOI: 10.1128/AAC.00050-10
BindingDB Entry DOI: 10.7270/Q2348KM2 |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 6
(Homo sapiens (Human)) | BDBM50335523

((6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-y...)Show SMILES CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16| Show InChI InChI=1S/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/b20-9+/t10-,14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PAH uptake in OAT1-expressing S2 cells |
Eur J Pharmacol 438: 137-42 (2002)
BindingDB Entry DOI: 10.7270/Q2N87C2C |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 11
(Homo sapiens (Human)) | BDBM50335523

((6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-y...)Show SMILES CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16| Show InChI InChI=1S/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/b20-9+/t10-,14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of Estrone sulfate uptake in OAT4-expressing S2 cells |
Eur J Pharmacol 438: 137-42 (2002)
BindingDB Entry DOI: 10.7270/Q2N87C2C |
More data for this
Ligand-Target Pair | |
Oligopeptide transporter, kidney isoform
(Rattus norvegicus) | BDBM50335523

((6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-y...)Show SMILES CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16| Show InChI InChI=1S/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/b20-9+/t10-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biozentrum of the Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of Gly-Sar uptake (pH6.0) in SKPT cells |
Eur J Pharm Biopharm 59: 17-24 (2004)
Article DOI: 10.1016/j.ejpb.2004.07.008
BindingDB Entry DOI: 10.7270/Q2TT4S7H |
More data for this
Ligand-Target Pair | |
Solute carrier family 15 member 2
(Homo sapiens (Human)) | BDBM50335523

((6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-y...)Show SMILES CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16| Show InChI InChI=1S/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/b20-9+/t10-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Binding affinity to human PepT2 in SKTP cells |
Bioorg Med Chem 19: 4544-51 (2011)
Article DOI: 10.1016/j.bmc.2011.06.027
BindingDB Entry DOI: 10.7270/Q2MP549P |
More data for this
Ligand-Target Pair | |
Oligopeptide transporter small intestine isoform
(Homo sapiens (Human)) | BDBM50335523

((6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-y...)Show SMILES CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16| Show InChI InChI=1S/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/b20-9+/t10-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biozentrum of the Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of Gly-Sar uptake (pH6.0) in Caco-2 cells |
Eur J Pharm Biopharm 59: 17-24 (2004)
Article DOI: 10.1016/j.ejpb.2004.07.008
BindingDB Entry DOI: 10.7270/Q2TT4S7H |
More data for this
Ligand-Target Pair | |
Oligopeptide transporter small intestine isoform
(Homo sapiens (Human)) | BDBM50335523

((6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-y...)Show SMILES CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16| Show InChI InChI=1S/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/b20-9+/t10-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
| Assay Description
Binding affinity against membrane transport protein PEPT1 in human Caco-2 cells |
J Med Chem 48: 4410-9 (2005)
Article DOI: 10.1021/jm048982w
BindingDB Entry DOI: 10.7270/Q2Q24116 |
More data for this
Ligand-Target Pair | |
Serum albumin
(Homo sapiens (Human)) | BDBM50335523

((6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-y...)Show SMILES CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16| Show InChI InChI=1S/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/b20-9+/t10-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a |
CNRS-CPBS/UMR 5236
Curated by ChEMBL
| Assay Description
Binding affinity to first site on human serum albumin by SPR |
Antimicrob Agents Chemother 53: 1528-31 (2009)
Article DOI: 10.1128/AAC.00971-08
BindingDB Entry DOI: 10.7270/Q2SN09ZK |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 7
(Rattus norvegicus) | BDBM50335523

((6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-y...)Show SMILES CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16| Show InChI InChI=1S/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/b20-9+/t10-,14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PGF2alpha in OAT2-S2 cells |
Eur J Pharmacol 465: 1-7 (2003)
BindingDB Entry DOI: 10.7270/Q2CC11SX |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 7
(Homo sapiens (Human)) | BDBM50335523

((6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-y...)Show SMILES CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16| Show InChI InChI=1S/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/b20-9+/t10-,14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.68E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PGF2alpha in OAT2-S2 cells |
Eur J Pharmacol 465: 1-7 (2003)
BindingDB Entry DOI: 10.7270/Q2CC11SX |
More data for this
Ligand-Target Pair | |
Gcn5-related N-acetyltransferases (GNAT) superfamily protein PA4794 (PA4794)
(Pseudomonas aeruginosa) | BDBM50335523

((6R,7R)-3-(acetoxymethyl)-7-(2-(2-aminothiazol-4-y...)Show SMILES CO\N=C(\C(=O)N[C@H]1[C@H]2SCC(COC(C)=O)=C(N2C1=O)C(O)=O)c1csc(N)n1 |r,c:16| Show InChI InChI=1S/C16H17N5O7S2/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26)/b20-9+/t10-,14-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | 7.5 | 25 |
University of Virginia
| Assay Description
Isothermal titration calorimetry (ITC) measurements were performed at 25
°C using an iTC200 calorimeter (MicroCal). Preparations of
purified pr... |
J Biol Chem 288: 30223-35 (2013)
Article DOI: 10.1074/jbc.M113.501353
BindingDB Entry DOI: 10.7270/Q22N514W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
