

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50337282 3-[N-(4-acetylphenyl)amino]benzoic acid::CHEMBL1682201::US9271961, 6
SMILES: CC(=O)c1ccc(Nc2cccc(c2)C(O)=O)cc1
InChI Key: InChIKey=WVJCBUXADPESMP-UHFFFAOYSA-N
Data: 17 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C4 (AK1C4) (Homo sapiens (Human)) | BDBM50337282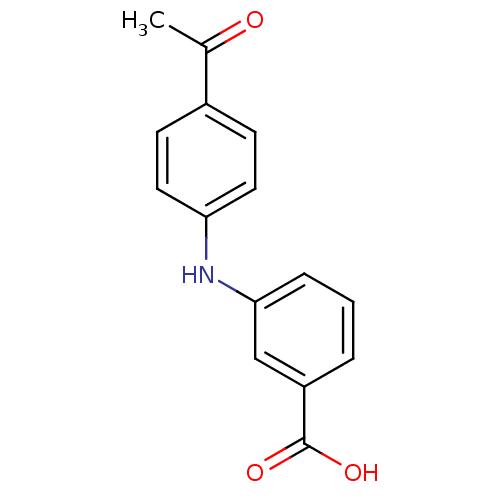 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in baculovirus infected SF-21 cells assessed as formation of PGH2 from PGG2 using arachidonic acid as substrate preincub... | J Med Chem 55: 2311-23 (2012) Article DOI: 10.1021/jm201547v BindingDB Entry DOI: 10.7270/Q2C24XGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldose reductase (AR) (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family member 1B10 (AKR1B10) (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-1 (COX-1) (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-Hydroxysteroid Dehydrogenase 5 (17-beta-HSD5, AKR1C3) (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of AKR1C3 by fluorimetric method | Bioorg Med Chem Lett 21: 1464-8 (2011) Article DOI: 10.1016/j.bmcl.2011.01.010 BindingDB Entry DOI: 10.7270/Q24J0FD4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of AKR1C2 by fluorimetric method | Bioorg Med Chem Lett 21: 1464-8 (2011) Article DOI: 10.1016/j.bmcl.2011.01.010 BindingDB Entry DOI: 10.7270/Q24J0FD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C1 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assay | J Med Chem 55: 2311-23 (2012) Article DOI: 10.1021/jm201547v BindingDB Entry DOI: 10.7270/Q2C24XGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (AK1C4) (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C4 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assay | J Med Chem 55: 2311-23 (2012) Article DOI: 10.1021/jm201547v BindingDB Entry DOI: 10.7270/Q2C24XGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-Hydroxysteroid Dehydrogenase 5 (17-beta-HSD5, AKR1C3) (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assay | J Med Chem 55: 2311-23 (2012) Article DOI: 10.1021/jm201547v BindingDB Entry DOI: 10.7270/Q2C24XGP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assay | J Med Chem 55: 2311-23 (2012) Article DOI: 10.1021/jm201547v BindingDB Entry DOI: 10.7270/Q2C24XGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family member 1B10 (AKR1B10) (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1B10 assessed as NADP+ dependent reduction of DL-glyceraldehyde by fluorescence assay | J Med Chem 55: 2311-23 (2012) Article DOI: 10.1021/jm201547v BindingDB Entry DOI: 10.7270/Q2C24XGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldose reductase (AR) (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1B1 assessed as NADP+ dependent reduction of DL-glyceraldehyde by fluorescence assay | J Med Chem 55: 2311-23 (2012) Article DOI: 10.1021/jm201547v BindingDB Entry DOI: 10.7270/Q2C24XGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-Hydroxysteroid Dehydrogenase 5 (17-beta-HSD5, AKR1C3) (Homo sapiens (Human)) | BDBM50337282 (3-[N-(4-acetylphenyl)amino]benzoic acid | CHEMBL16...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||