

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50338736 (+/-)-(3R,4S)-6-(3-chloro-1H-indol-7-yl)-5,7-difluoro-2,2,4-trimethyl-1,2,3,4-tetrahydroquinolin-3-yl cyclohexylcarbamate::CHEMBL1684344
SMILES: C[C@@H]1[C@@H](OC(=O)NC2CCCCC2)C(C)(C)Nc2cc(F)c(c(F)c12)-c1cccc2c(Cl)c[nH]c12
InChI Key: InChIKey=BQZKKRYIQPCEKF-HWRSSNJWSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50338736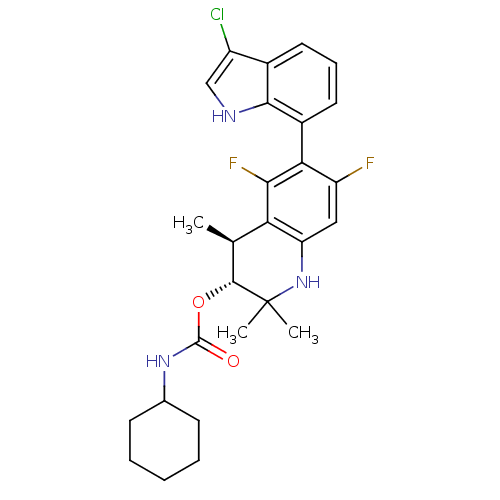 ((+/-)-(3R,4S)-6-(3-chloro-1H-indol-7-yl)-5,7-diflu...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled dexamethasone from GR | Bioorg Med Chem Lett 21: 1658-62 (2011) Article DOI: 10.1016/j.bmcl.2011.01.106 BindingDB Entry DOI: 10.7270/Q2VQ32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50338736 ((+/-)-(3R,4S)-6-(3-chloro-1H-indol-7-yl)-5,7-diflu...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | Bioorg Med Chem Lett 21: 1658-62 (2011) Article DOI: 10.1016/j.bmcl.2011.01.106 BindingDB Entry DOI: 10.7270/Q2VQ32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50338736 ((+/-)-(3R,4S)-6-(3-chloro-1H-indol-7-yl)-5,7-diflu...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to mineralocorticoid receptor | Bioorg Med Chem Lett 21: 1658-62 (2011) Article DOI: 10.1016/j.bmcl.2011.01.106 BindingDB Entry DOI: 10.7270/Q2VQ32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50338736 ((+/-)-(3R,4S)-6-(3-chloro-1H-indol-7-yl)-5,7-diflu...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Transrepression activity at GR expressed in IL-1beta- and TNFalpha-stimulated HepG2 cells assessed as inhibition of NFKB- or AP-1 mediated E-selectin... | Bioorg Med Chem Lett 21: 1658-62 (2011) Article DOI: 10.1016/j.bmcl.2011.01.106 BindingDB Entry DOI: 10.7270/Q2VQ32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50338736 ((+/-)-(3R,4S)-6-(3-chloro-1H-indol-7-yl)-5,7-diflu...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 435 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GR expressed in rat H4IIEC3 cells assessed as induction of PEPCK transactivation by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 1658-62 (2011) Article DOI: 10.1016/j.bmcl.2011.01.106 BindingDB Entry DOI: 10.7270/Q2VQ32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50338736 ((+/-)-(3R,4S)-6-(3-chloro-1H-indol-7-yl)-5,7-diflu...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GR expressed in african green monkey CV1 cells transfected with luciferase gene linked to MMTV promoter assessed as induction of ... | Bioorg Med Chem Lett 21: 1658-62 (2011) Article DOI: 10.1016/j.bmcl.2011.01.106 BindingDB Entry DOI: 10.7270/Q2VQ32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50338736 ((+/-)-(3R,4S)-6-(3-chloro-1H-indol-7-yl)-5,7-diflu...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Transrepression activity at GR expressed in NHDF cells assessed as IL-1beta-mediated IL-6 transcription by ELISA | Bioorg Med Chem Lett 21: 1658-62 (2011) Article DOI: 10.1016/j.bmcl.2011.01.106 BindingDB Entry DOI: 10.7270/Q2VQ32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||