

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50339607 1-Hydroxy-6-phenyl-4-trifluoromethyl-1H-indole-2-carboxylic Acid::CHEMBL1688790::LDHA Inhibitor, 4
SMILES: OC(=O)c1cc2c(cc(cc2n1O)-c1ccccc1)C(F)(F)F
InChI Key: InChIKey=KGXDBNBXAPVUIM-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactate dehydrogenase A (LDHA) (Homo sapiens (Human)) | BDBM50339607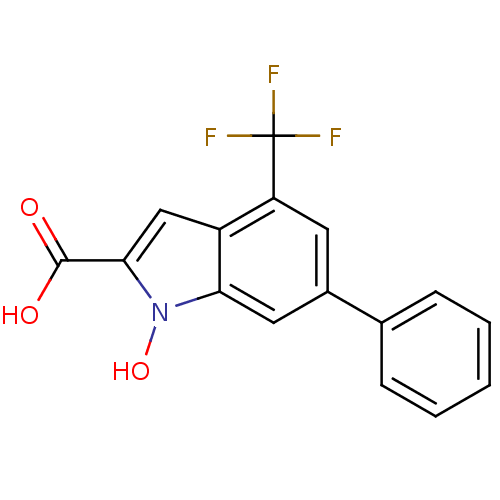 (1-Hydroxy-6-phenyl-4-trifluoromethyl-1H-indole-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` di Pisa Curated by ChEMBL | Assay Description Competitive inhibition of human LDH-A using pyruvate as substrate after 5 mins by calorimetric assay relative to control | J Med Chem 54: 1599-612 (2011) Article DOI: 10.1021/jm101007q BindingDB Entry DOI: 10.7270/Q2J38SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactate dehydrogenase A (LDHA) (Homo sapiens (Human)) | BDBM50339607 (1-Hydroxy-6-phenyl-4-trifluoromethyl-1H-indole-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` di Pisa Curated by ChEMBL | Assay Description Competitive inhibition of human LDH-A using NADH as substrate after 5 mins by calorimetric assay relative to control | J Med Chem 54: 1599-612 (2011) Article DOI: 10.1021/jm101007q BindingDB Entry DOI: 10.7270/Q2J38SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactate dehydrogenase A (LDHA) (Homo sapiens (Human)) | BDBM50339607 (1-Hydroxy-6-phenyl-4-trifluoromethyl-1H-indole-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca | Assay Description Enzyme assay using lactate dehydrogenase A (LDHA). | J Med Chem 55: 3285-306 (2012) Article DOI: 10.1021/jm201734r BindingDB Entry DOI: 10.7270/Q21J9896 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactate dehydrogenase A (LDHA) (Homo sapiens (Human)) | BDBM50339607 (1-Hydroxy-6-phenyl-4-trifluoromethyl-1H-indole-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca | Assay Description NMR spectra were acquired on Bruker Avance 600 MHz spectrometers at 298 K using a 5 mm triple-resonance HCN cryoprobe. Ligand binding was detected u... | J Med Chem 55: 3285-306 (2012) Article DOI: 10.1021/jm201734r BindingDB Entry DOI: 10.7270/Q21J9896 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||