

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50340918 4-(4-Bromo-2-fluoroanilino)-6,7-dimethoxyquinazoline::CHEMBL1277620::N-(4-bromo-2-fluorophenyl)-6,7-dimethoxyquinazolin-4-amine
SMILES: COc1cc2ncnc(Nc3ccc(Br)cc3F)c2cc1OC
InChI Key: InChIKey=VHFBRSZCMAZULY-UHFFFAOYSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50340918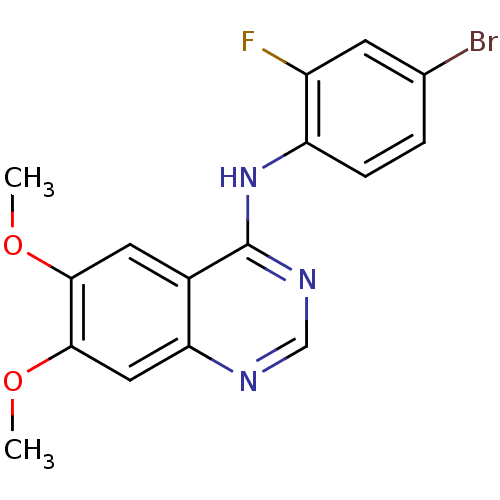 (4-(4-Bromo-2-fluoroanilino)-6,7-dimethoxyquinazoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human KDR expressed in insect Sf21 cells preincubated for 15 mins followed by substrate addition measured after ... | Eur J Med Chem 112: 20-32 (2016) BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50340918 (4-(4-Bromo-2-fluoroanilino)-6,7-dimethoxyquinazoli...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-1 heavy chain/ Tyrosine-protein kinase receptor RET (Homo sapiens (Human)) | BDBM50340918 (4-(4-Bromo-2-fluoroanilino)-6,7-dimethoxyquinazoli...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of KIF5B/RET (unknown origin) expressed in mouse BA/F3 cells assessed as reduction in cell viability after 48 hrs by Cell titre glo-based ... | Eur J Med Chem 112: 20-32 (2016) BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50340918 (4-(4-Bromo-2-fluoroanilino)-6,7-dimethoxyquinazoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France Curated by ChEMBL | Assay Description Inhibition of recombinant human VEGFR-2 using poly(Glu4/Tyr) and [gamma32P]ATP as substrate after 1 hr by scintillation counting | J Med Chem 55: 1189-204 (2012) Article DOI: 10.1021/jm2013453 BindingDB Entry DOI: 10.7270/Q2086695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50340918 (4-(4-Bromo-2-fluoroanilino)-6,7-dimethoxyquinazoli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ Lille Nord de France, F-59000 Lille, France; UDSL, ICPAL, EA 4481, F-59006 Lille, France. Curated by ChEMBL | Assay Description Inhibition of human EGFR-mediated poly(Glu4Tyr) phosphorylation after 1 hr | Bioorg Med Chem Lett 21: 2106-12 (2011) Article DOI: 10.1016/j.bmcl.2011.01.137 BindingDB Entry DOI: 10.7270/Q2C24WRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50340918 (4-(4-Bromo-2-fluoroanilino)-6,7-dimethoxyquinazoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ Lille Nord de France, F-59000 Lille, France; UDSL, ICPAL, EA 4481, F-59006 Lille, France. Curated by ChEMBL | Assay Description Inhibition of recombinant human VEGFR-2-mediated poly(Glu4Tyr) phosphorylation after 1 hr | Bioorg Med Chem Lett 21: 2106-12 (2011) Article DOI: 10.1016/j.bmcl.2011.01.137 BindingDB Entry DOI: 10.7270/Q2C24WRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50340918 (4-(4-Bromo-2-fluoroanilino)-6,7-dimethoxyquinazoli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Nord de France Curated by ChEMBL | Assay Description Inhibition of human EGFR expressed in human A431 cells using poly(Glu4/Tyr) and [gamma32P]ATP as substrate after 1 hr by scintillation counting | J Med Chem 55: 1189-204 (2012) Article DOI: 10.1021/jm2013453 BindingDB Entry DOI: 10.7270/Q2086695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50340918 (4-(4-Bromo-2-fluoroanilino)-6,7-dimethoxyquinazoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of KDR (unknown origin) expressed in mouse BA/F3 cells assessed as reduction in cell viability after 48 hrs by Cell titre glo-based lumine... | Eur J Med Chem 112: 20-32 (2016) BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||