

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50341155 3-(anthracen-9-yl)-5-p-tolyl-4,5-dihydro-1H-pyrazole::CHEMBL1760716
SMILES: Cc1ccc(cc1)C1CC(N=N1)c1c2ccccc2cc2ccccc12
InChI Key: InChIKey=ORDSRUWWRNHCAJ-UHFFFAOYSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50341155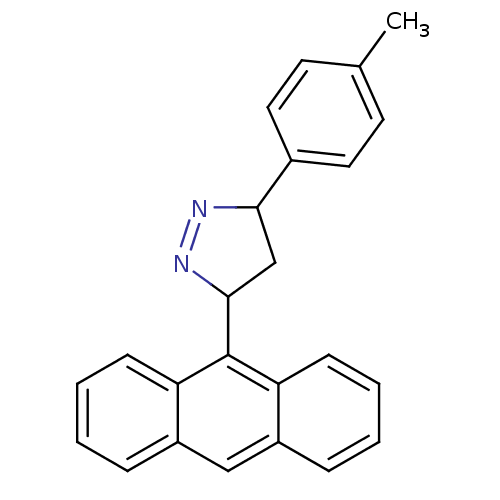 (3-(anthracen-9-yl)-5-p-tolyl-4,5-dihydro-1H-pyrazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine Oxidase Type B (MAO-B) (Rattus norvegicus (rat)) | BDBM50341155 (3-(anthracen-9-yl)-5-p-tolyl-4,5-dihydro-1H-pyrazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50341155 (3-(anthracen-9-yl)-5-p-tolyl-4,5-dihydro-1H-pyrazo...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine oxidase (Rattus norvegicus (rat)) | BDBM50341155 (3-(anthracen-9-yl)-5-p-tolyl-4,5-dihydro-1H-pyrazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||