

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50341684 6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis(4-methylpyridin-2-amine)::CHEMBL1766633::NOS Inhibitor, 3h::US10759791, Compound 31a::US9951014, Name 31a
SMILES: Cc1cc(N)nc(CCc2cccc(CCc3cc(C)cc(N)n3)c2)c1
InChI Key: InChIKey=GBBKOTDCCCGYNX-UHFFFAOYSA-N
Data: 12 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50341684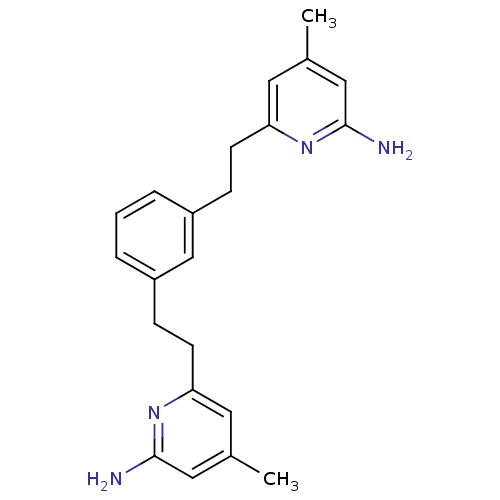 (6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, Irvine | Assay Description Inhibition assay using nitric oxide synthases. | Biochemistry 49: 10803-10 (2010) Article DOI: 10.1021/bi1013479 BindingDB Entry DOI: 10.7270/Q2KS6Q51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50341684 (6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli by UV-vis spectrometric analysis | J Med Chem 54: 2039-48 (2011) Article DOI: 10.1021/jm101071n BindingDB Entry DOI: 10.7270/Q2571CB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50341684 (6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Enzyme inhibition was evaluated by measuring NO production with the hemoglobin capture assay, which was performed with purified NOSs in 96-well plate... | US Patent US10759791 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50341684 (6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex | Assay Description The NOSs isoform assays involved subjecting 3-8 to an oxyhemoglobin NO capture assay using a Biotek Gen5™ microplate reader. IC50 values for each com... | J Med Chem 52: 379-88 (2009) BindingDB Entry DOI: 10.7270/Q21N83G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50341684 (6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 682 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Enzyme inhibition was evaluated by measuring NO production with the hemoglobin capture assay, which was performed with purified NOSs in 96-well plate... | US Patent US10759791 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50341684 (6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 682 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex | Assay Description The NOSs isoform assays involved subjecting 3-8 to an oxyhemoglobin NO capture assay using a Biotek Gen5™ microplate reader. IC50 values for each com... | J Med Chem 52: 379-88 (2009) BindingDB Entry DOI: 10.7270/Q21N83G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric Oxide Synthase, inducible (Mus musculus (mouse)) | BDBM50341684 (6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 682 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse macrophage recombinant iNOS expressed in Escherichia coli by UV-vis spectrometric analysis | J Med Chem 54: 2039-48 (2011) Article DOI: 10.1021/jm101071n BindingDB Entry DOI: 10.7270/Q2571CB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric Oxide Synthase, endothelial (Bos taurus (bovine)) | BDBM50341684 (6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli by UV-vis spectrometric analysis | J Med Chem 54: 2039-48 (2011) Article DOI: 10.1021/jm101071n BindingDB Entry DOI: 10.7270/Q2571CB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric-oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50341684 (6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Enzyme inhibition was evaluated by measuring NO production with the hemoglobin capture assay, which was performed with purified NOSs in 96-well plate... | US Patent US10759791 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50341684 (6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex | Assay Description The NOSs isoform assays involved subjecting 3-8 to an oxyhemoglobin NO capture assay using a Biotek Gen5™ microplate reader. IC50 values for each com... | J Med Chem 52: 379-88 (2009) BindingDB Entry DOI: 10.7270/Q21N83G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50341684 (6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, Irvine | Assay Description Inhibition assay using nitric oxide synthases. | Biochemistry 49: 10803-10 (2010) Article DOI: 10.1021/bi1013479 BindingDB Entry DOI: 10.7270/Q2KS6Q51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50341684 (6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, Irvine | Assay Description Inhibition assay using nitric oxide synthases. | Biochemistry 49: 10803-10 (2010) Article DOI: 10.1021/bi1013479 BindingDB Entry DOI: 10.7270/Q2KS6Q51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||