

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50341779 4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carbonyl)-amino]phenyl}piperazine-1-carbonyl)-trans-cyclohexanecarboxylic Acid::CHEMBL1766809
SMILES: OC(=O)[C@H]1CC[C@@H](CC1)C(=O)N1CCN(CC1)c1ccc(NC(=O)c2nc(oc2C(F)(F)F)-c2ccccc2)cc1
InChI Key: InChIKey=LEHBYBLXUUUJMC-MXVIHJGJSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diacylglycerol O-acyltransferase 1 (DGAT1) (Homo sapiens (Human)) | BDBM50341779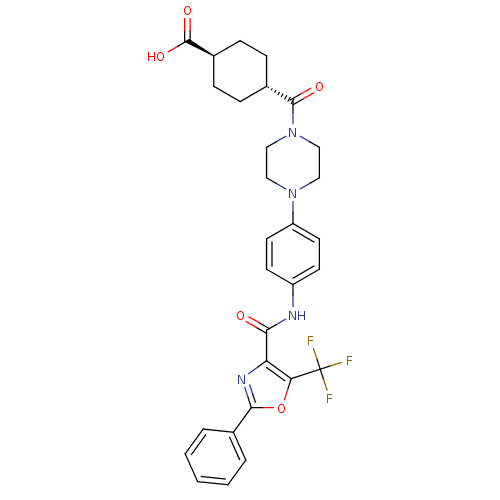 (4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (DGAT1) (Homo sapiens (Human)) | BDBM50341779 (4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in CHO-K1 cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||