

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50343142 CHEMBL1773168::N-(4-butylphenyl)-2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamide
SMILES: CCCCc1ccc(NS(=O)(=O)c2ccc3NC(=O)c4cccc2c34)cc1
InChI Key: InChIKey=JPWASJCSEDYSDE-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein Tyrosine Phosphatase PTPB (Mycobacterium tuberculosis) | BDBM50343142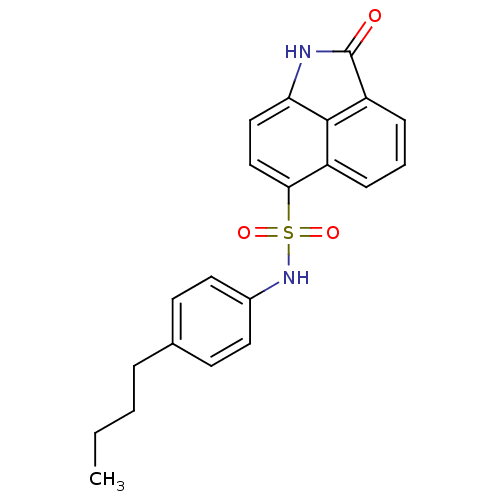 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Non-competitive inhibition of Mycobacterium tuberculosis N-terminal His6-tagged PTPB assessed as production of p-nitrophenol from pNPP substrate by L... | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tyrosine Phosphatase PTPB (Mycobacterium tuberculosis) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis PTPB expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human SHP2 expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic cell protein-tyrosine phosphatase 70Z-PEP (Homo sapiens (Human)) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human Lyp expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fas-associated protein-tyrosine phosphatase 1 (FAP1) (Homo sapiens (Human)) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human FAP1 expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase MEG2 (PTP-Meg2) (Homo sapiens (Human)) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human MEG2 expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase F (LAR) (Homo sapiens (Human)) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human LAR expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase alpha (Homo sapiens (Human)) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human PTPalpha expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase (VHR) (Homo sapiens (Human)) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human VHR expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 6 (Homo sapiens (Human)) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human MKP3 expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 1 (Homo sapiens (Human)) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human PRL1 expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 4A3 (Homo sapiens (Human)) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human PRL3 expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase CDC14A (Homo sapiens (Human)) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human Cdc14A expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tyrosine Phosphatase PTPB (Mycobacterium tuberculosis) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis N-terminal His6-tagged PTPB | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (PTPβ) (Homo sapiens (Human)) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human PTPB expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tyrosine Phosphatase PTPB (Mycobacterium tuberculosis) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis N-terminal His6-tagged PTPB preincubated with compound for 30 mins before pNPP substrate addition by micropl... | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable low molecular weight protein-tyrosine-phosphatase (Mycobacterium tuberculosis) | BDBM50343142 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis PTPA expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||