

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50346463 (E)-2-chloro-3'-fluoro-4,4'-dihydroxybiphenyl-3-carbaldehyde oxime::CHEMBL1782958
SMILES: Oc1ccc(cc1F)-c1ccc(O)c(CN=O)c1Cl
InChI Key: InChIKey=LJPIWESQQAGJHE-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol receptor beta (ERβ) (Homo sapiens (Human)) | BDBM50346463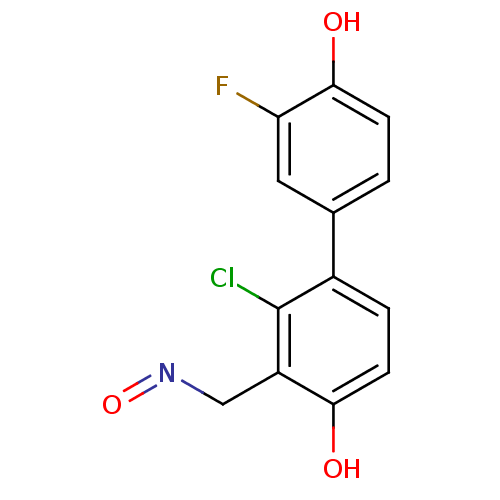 ((E)-2-chloro-3'-fluoro-4,4'-dihydroxybiphenyl-3-ca...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human full-length ERbeta receptor by competitive radiometric binding assay | Eur J Med Chem 46: 2453-62 (2011) Article DOI: 10.1016/j.ejmech.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZC8362 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50346463 ((E)-2-chloro-3'-fluoro-4,4'-dihydroxybiphenyl-3-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Pisa Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1511-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.057 BindingDB Entry DOI: 10.7270/Q2891765 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50346463 ((E)-2-chloro-3'-fluoro-4,4'-dihydroxybiphenyl-3-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | 5.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Pisa Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1511-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.057 BindingDB Entry DOI: 10.7270/Q2891765 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50346463 ((E)-2-chloro-3'-fluoro-4,4'-dihydroxybiphenyl-3-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | 7.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Pisa Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1511-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.057 BindingDB Entry DOI: 10.7270/Q2891765 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50346463 ((E)-2-chloro-3'-fluoro-4,4'-dihydroxybiphenyl-3-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | 7.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Pisa Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins by CO2 hydration stopped-flow assay | Bioorg Med Chem 21: 1511-5 (2013) Article DOI: 10.1016/j.bmc.2012.08.057 BindingDB Entry DOI: 10.7270/Q2891765 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50346463 ((E)-2-chloro-3'-fluoro-4,4'-dihydroxybiphenyl-3-ca...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Agonist activity at human full-length ERalpha receptor expressed in human HEC1 cells co-expressing (ERE)2-pS2-luc gene assessed as transcriptional ac... | Eur J Med Chem 46: 2453-62 (2011) Article DOI: 10.1016/j.ejmech.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZC8362 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estradiol receptor beta (ERβ) (Homo sapiens (Human)) | BDBM50346463 ((E)-2-chloro-3'-fluoro-4,4'-dihydroxybiphenyl-3-ca...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Agonist activity at human full-length ERbeta receptor expressed in human HEC1 cells co-expressing (ERE)2-pS2-luc gene assessed as transcriptional act... | Eur J Med Chem 46: 2453-62 (2011) Article DOI: 10.1016/j.ejmech.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZC8362 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||