 Found 7 hits for monomerid = 50346585
Found 7 hits for monomerid = 50346585 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346585
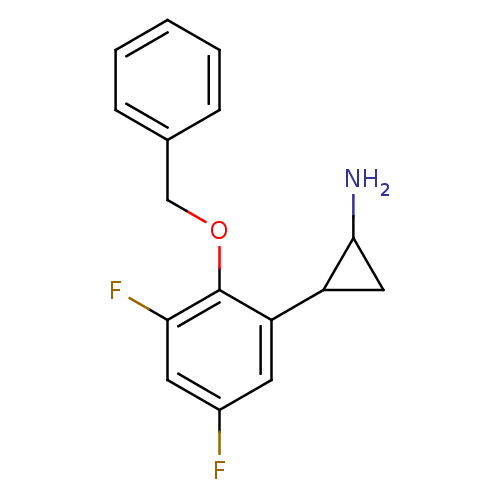
(CHEMBL1795980 | S2101)Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346585

(CHEMBL1795980 | S2101)Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
Bioorg Med Chem 19: 3625-36 (2011)
Article DOI: 10.1016/j.bmc.2011.01.046
BindingDB Entry DOI: 10.7270/Q23X870S |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346585

(CHEMBL1795980 | S2101)Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton
Curated by ChEMBL
| Assay Description
Inhibition of human LSD1 |
Bioorg Med Chem 19: 3709-16 (2011)
Article DOI: 10.1016/j.bmc.2011.02.017
BindingDB Entry DOI: 10.7270/Q2J38SWT |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50346585

(CHEMBL1795980 | S2101)Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO B |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Amine oxidase (flavin-containing) A
(Homo sapiens (Human)) | BDBM50346585

(CHEMBL1795980 | S2101)Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MAO A |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346585

(CHEMBL1795980 | S2101)Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LSD1 using dimethylated H3K4 peptide as substrate after 1 hr |
J Med Chem 56: 9496-508 (2014)
Article DOI: 10.1021/jm400870h
BindingDB Entry DOI: 10.7270/Q2Z60QJ7 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346585

(CHEMBL1795980 | S2101)Show InChI InChI=1S/C16H15F2NO/c17-11-6-13(12-8-15(12)19)16(14(18)7-11)20-9-10-4-2-1-3-5-10/h1-7,12,15H,8-9,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Systems and Structural Biology Center
| Assay Description
The kinetic inhibition parameters of LSD1 demethylase inhibition were obtained using the peroxidase-coupled reaction method. |
Biochemistry 49: 6494-503 (2010)
Article DOI: 10.1021/bi100299r
BindingDB Entry DOI: 10.7270/Q2PK0DSZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data