

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50347546 CHEMBL1801951::US10434085, Compound LL-Z164-1
SMILES: COc1cc(O)c2c(c1)\C=C\C[C@H](O)[C@H](O)C(=O)CCC[C@H](C)OC2=O
InChI Key: InChIKey=WFIUAGOQGGAREU-XZUJVHTDSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase kinase kinase 7 (Homo sapiens (Human)) | BDBM50347546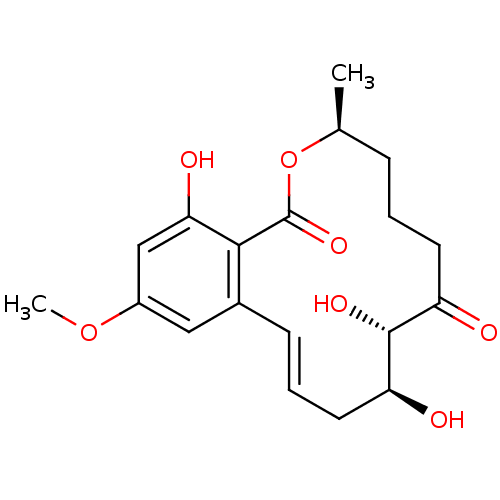 (CHEMBL1801951 | US10434085, Compound LL-Z164-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Greensboro Curated by ChEMBL | Assay Description Inhibition of TAK1 (unknown origin) | Bioorg Med Chem 23: 6993-9 (2015) BindingDB Entry DOI: 10.7270/Q25D8TNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7 (Homo sapiens (Human)) | BDBM50347546 (CHEMBL1801951 | US10434085, Compound LL-Z164-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Greensboro US Patent | Assay Description The assay was performed at BSP Biosience Inc. (5Z)-7-oxozeaenol was used as a positive control. Analogue 3 was not tested due to the short-term stabi... | US Patent US10434085 (2019) BindingDB Entry DOI: 10.7270/Q2SB483C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B (Homo sapiens (Human)) | BDBM50347546 (CHEMBL1801951 | US10434085, Compound LL-Z164-1) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Greensboro Curated by ChEMBL | Assay Description Inhibition of NFkappa p65 isolated from nuclear extract of human HeLa cells by ELISA | J Nat Prod 74: 1126-31 (2011) Article DOI: 10.1021/np200062x BindingDB Entry DOI: 10.7270/Q25M6621 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||