 Found 11 hits for monomerid = 50348452
Found 11 hits for monomerid = 50348452 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50348452
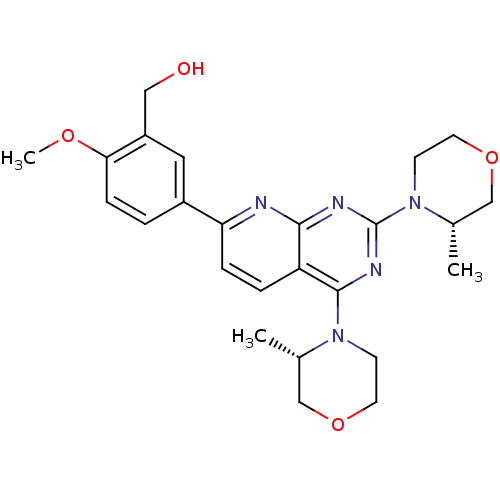
(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED
US Patent
| Assay Description
The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... |
US Patent US9102670 (2015)
BindingDB Entry DOI: 10.7270/Q26M35MZ |
More data for this
Ligand-Target Pair | |
Ferrochelatase R115L (FECH)
(Homo sapiens (Human)) | BDBM50348452

(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... |
ACS Chem Biol 11: 1245-54 (2016)
Article DOI: 10.1021/acschembio.5b01063
BindingDB Entry DOI: 10.7270/Q2FB51R8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50348452

(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 4308-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.099
BindingDB Entry DOI: 10.7270/Q2MG7PV5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50348452

(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FLAG-tagged mTOR (1362 to 2549) (unknown origin) expressed in HEK293 cells |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50348452

(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 23: 1212-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.019
BindingDB Entry DOI: 10.7270/Q2N29Z97 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50348452

(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 (unknown origin) |
J Med Chem 61: 4656-4687 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01019 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50348452

(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal FLAG-tagged mTOR (1362-end residues) in presence of [gamma33P]ATP after 40 mins |
Eur J Med Chem 129: 135-150 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.015
BindingDB Entry DOI: 10.7270/Q2QN692K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50348452

(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 peptide substrate measured after 1 hr by Lance Ultra assay |
Bioorg Med Chem 26: 3992-4000 (2018)
Article DOI: 10.1016/j.bmc.2018.06.025
BindingDB Entry DOI: 10.7270/Q2668GVK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50348452

(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate measured after 1 hr by Kinase-Glo luminescent assay |
Bioorg Med Chem 26: 3992-4000 (2018)
Article DOI: 10.1016/j.bmc.2018.06.025
BindingDB Entry DOI: 10.7270/Q2668GVK |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50348452

(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 (unknown origin) |
J Med Chem 61: 4656-4687 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01019 |
More data for this
Ligand-Target Pair | |
p110α/p85α
(Homo sapiens (Human)) | BDBM50348452

(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha using phosphatidylinositol 4,5-bisphosphate as substrate after 30 mins by HTRF assay |
Eur J Med Chem 129: 135-150 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.015
BindingDB Entry DOI: 10.7270/Q2QN692K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data