

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50349649 CHEMBL1809008
SMILES: O=C(Nc1ccccc1)N1C[C@@H](C=C2[C@H]1Cc1c[nH]c3cccc2c13)C(=O)N1CCOCC1
InChI Key: InChIKey=YLMYBTJRYYSEKF-WZONZLPQSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| chemokine (C-X-C motif) receptor 3 (Homo sapiens (Human)) | BDBM50349649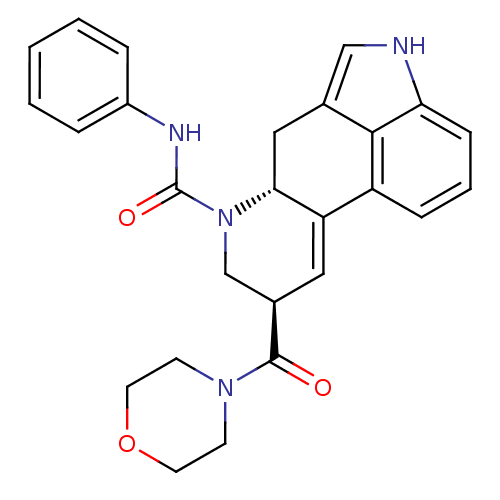 (CHEMBL1809008) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of radiolabeled CXCL11 from human CXCR3 expressed in CHO cells by scintillation proximity assay | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50349649 (CHEMBL1809008) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at CXCR3 in rat leukocytes assessed as inhibition of ITAC-induced cell migration by flow cytometry | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50349649 (CHEMBL1809008) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 expressed in mouse L1.2 cells assessed as inhibition of ITAC-induced calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chemokine (C-X-C motif) receptor 3 (Homo sapiens (Human)) | BDBM50349649 (CHEMBL1809008) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in mouse L1.2 cells assessed as inhibition of ITAC-induced calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||