

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50349826 SYRINGARESINOL
SMILES: COc1cc(cc(OC)c1O)[C@H]1OC[C@H]2[C@@H]1CO[C@@H]2c1cc(OC)c(O)c(OC)c1
InChI Key: InChIKey=KOWMJRJXZMEZLD-HCIHMXRSSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forkhead box protein O3 (Homo sapiens (Human)) | BDBM50349826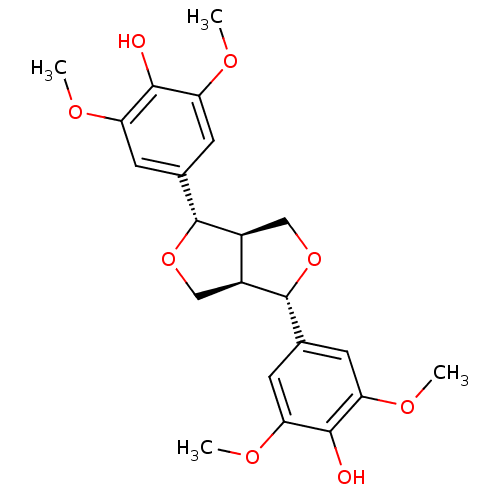 (SYRINGARESINOL) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.73E+6 | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Binding affinity to His-tagged FOXO3a (unknown origin) by SPR analysis | Bioorg Med Chem Lett 25: 307-9 (2014) Article DOI: 10.1016/j.bmcl.2014.11.045 BindingDB Entry DOI: 10.7270/Q2JH3NS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50349826 (SYRINGARESINOL) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Activation of PPARbeta-LBD (unknown origin) assessed as fluorescein-labeled coactivator C33 recruitment by TR-FRET assay | Bioorg Med Chem Lett 26: 3978-83 (2016) BindingDB Entry DOI: 10.7270/Q2WW7KMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50349826 (SYRINGARESINOL) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de S£o Carlos Curated by ChEMBL | Assay Description Inhibition of Leishmania amazonensis recombinant arginase expressed in Escherichia coli Rosetta (DE3) pLysS using L-arginine as substrate incubated f... | J Nat Prod 77: 392-6 (2014) Article DOI: 10.1021/np400717m BindingDB Entry DOI: 10.7270/Q2VX0J09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50349826 (SYRINGARESINOL) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal Plant Development Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA after 10 mins by ELISA reader | J Nat Prod 74: 1009-14 (2011) Article DOI: 10.1021/np100900k BindingDB Entry DOI: 10.7270/Q2TQ61WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50349826 (SYRINGARESINOL) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) assessed as p-nitrophenol release from pNPP substrate after 30 mins by spectrophotometry | J Nat Prod 76: 2080-7 (2013) Article DOI: 10.1021/np400533h BindingDB Entry DOI: 10.7270/Q23J3FFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50349826 (SYRINGARESINOL) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 2.76E+4 | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Binding affinity to recombinant PPARbeta-LBD (254 to 441 residues) (unknown origin) expressed in Escherichia coli Rosetta2 cells by isothermal titrat... | Bioorg Med Chem Lett 26: 3978-83 (2016) BindingDB Entry DOI: 10.7270/Q2WW7KMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||