

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50352717 CHEMBL1823213::US8835436, Example 151
SMILES: Cc1nc(C(=O)NC[C@@H](O)CN2CCN(CC2)c2cccc(C)c2C)c(C)n1-c1ccccc1
InChI Key: InChIKey=JEKNYMKFHWTAIS-XMMPIXPASA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50352717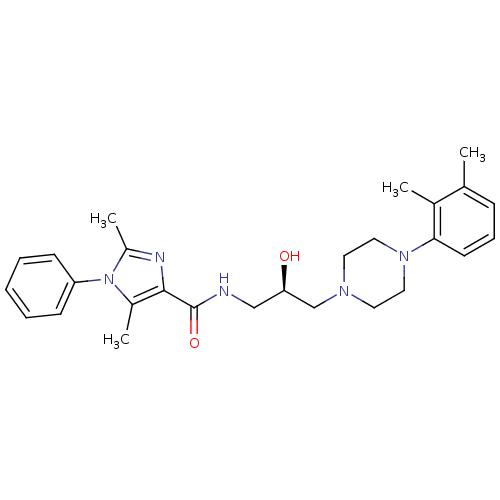 (CHEMBL1823213 | US8835436, Example 151) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 72.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Green Cross Corporation US Patent | Assay Description For serotonin 5-HT2A binding, an aliquot of human recombinant serotonin 5-HT2A receptor (PerkinElmer Life and Analytical Sciences, USA) expressed in ... | US Patent US8835436 (2014) BindingDB Entry DOI: 10.7270/Q24T6H2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50352717 (CHEMBL1823213 | US8835436, Example 151) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 227 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Green Cross Corporation US Patent | Assay Description For serotonin 5-HT2A binding, an aliquot of human recombinant serotonin 5-HT2A receptor (PerkinElmer Life and Analytical Sciences, USA) expressed in ... | US Patent US8835436 (2014) BindingDB Entry DOI: 10.7270/Q24T6H2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50352717 (CHEMBL1823213 | US8835436, Example 151) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]impiramine from human serotonin transporter membrane expressed in HEK293 cells | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50352717 (CHEMBL1823213 | US8835436, Example 151) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human recombinant serotonin 5-HT2A receptor expressed in CHO-K1 cells | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50352717 (CHEMBL1823213 | US8835436, Example 151) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50352717 (CHEMBL1823213 | US8835436, Example 151) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Green Cross Corporation US Patent | Assay Description For serotonin transporter binding assays, a reaction mixture with a final volume of 0.25 ml was prepared by mixing a test compound, human serotonin t... | US Patent US8835436 (2014) BindingDB Entry DOI: 10.7270/Q24T6H2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||