

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50353600 CHEMBL1829520
SMILES: CC(=O)OC[C@]12[C@H](C[C@@H]3[C@@H](OC(=O)c4ccccc4)[C@]1(OC3(C)C)[C@@](C)(O)C[C@H](OC(C)=O)[C@@H]2OC(C)=O)OC(=O)c1ccccc1
InChI Key: InChIKey=PNZVRNRYBVTQAP-OCRATEHUSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM50353600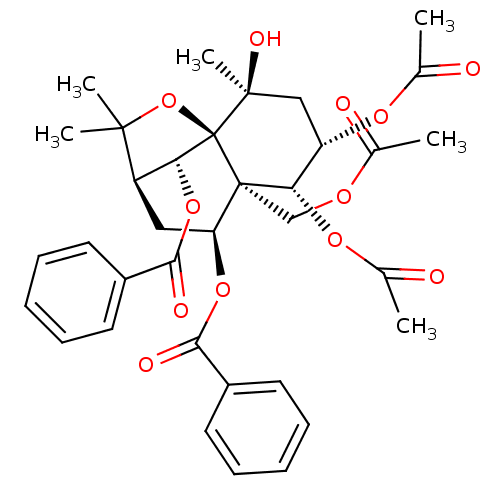 (CHEMBL1829520) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Universitario de Bio-Org£nica Antonio Gonz£lez Curated by ChEMBL | Assay Description Inhibition of human MDR1 expressed in mouse NIH-3T3 cells assessed as daunomycin efflux after 30 mins by flow cytometry | Eur J Med Chem 46: 4915-23 (2011) Article DOI: 10.1016/j.ejmech.2011.07.048 BindingDB Entry DOI: 10.7270/Q2N29XB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||