

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50355116 CHEMBL493923::US10278929, Compound 22
SMILES: Oc1ccccc1C1NC(=O)C(C#N)=C(SCc2ccccc2)S1
InChI Key: InChIKey=CBPDTXHHGBVEIJ-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transthyretin (Homo sapiens (Human)) | BDBM50355116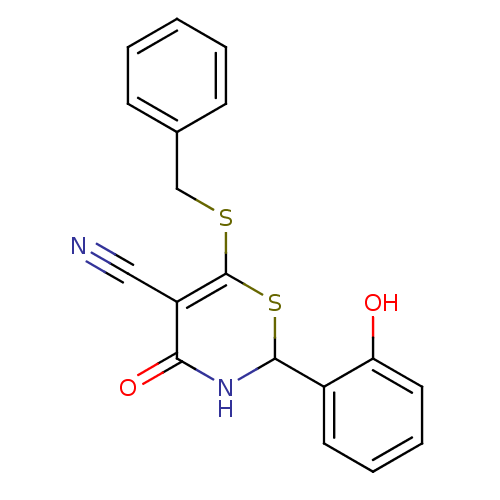 (CHEMBL493923 | US10278929, Compound 22) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description The FP assay was then adapted for HTS and used to screen ~120,000 small molecule library for compounds that displaced probe 5 from the T4 binding of ... | US Patent US8877795 (2014) BindingDB Entry DOI: 10.7270/Q2HT2N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transthyretin (Homo sapiens (Human)) | BDBM50355116 (CHEMBL493923 | US10278929, Compound 22) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The FP assay was then adapted for HTS and used to screen a ˜120,000 member small molecule library for compounds that displaced the FP probe from the ... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2H70J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galactokinase (GALK) (Homo sapiens (Human)) | BDBM50355116 (CHEMBL493923 | US10278929, Compound 22) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human galactokinase after 30 mins by Kinase-GloTM assay | ACS Med Chem Lett 2: 667-672 (2011) Article DOI: 10.1021/ml200131j BindingDB Entry DOI: 10.7270/Q251406Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase (Yersinia pestis) | BDBM50355116 (CHEMBL493923 | US10278929, Compound 22) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of His6-tagged Yersinia pestis KIM6 CDP-ME kinase assessed as ADP production after 30 mins by Kinase Glo luminescence-based assay or stand... | Bioorg Med Chem 19: 5886-95 (2011) Article DOI: 10.1016/j.bmc.2011.08.012 BindingDB Entry DOI: 10.7270/Q2W37WQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||