 Found 4 hits for monomerid = 50359195
Found 4 hits for monomerid = 50359195 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50359195
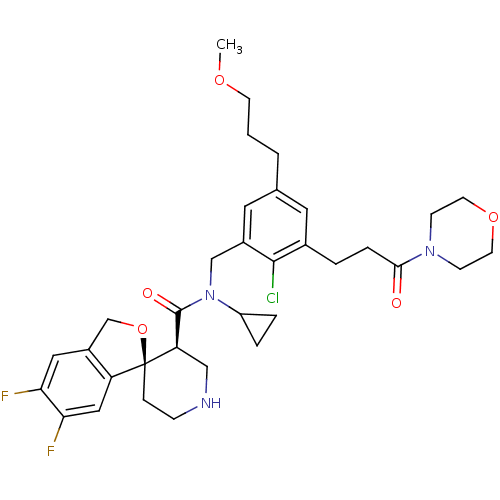
(CHEMBL1923132)Show SMILES COCCCc1cc(CCC(=O)N2CCOCC2)c(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@]22OCc3cc(F)c(F)cc23)c1 |r| Show InChI InChI=1S/C34H42ClF2N3O5/c1-43-12-2-3-22-15-23(4-7-31(41)39-10-13-44-14-11-39)32(35)24(16-22)20-40(26-5-6-26)33(42)28-19-38-9-8-34(28)27-18-30(37)29(36)17-25(27)21-45-34/h15-18,26,28,38H,2-14,19-21H2,1H3/t28-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 21: 7399-404 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.013
BindingDB Entry DOI: 10.7270/Q2PC32SW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50359195

(CHEMBL1923132)Show SMILES COCCCc1cc(CCC(=O)N2CCOCC2)c(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@]22OCc3cc(F)c(F)cc23)c1 |r| Show InChI InChI=1S/C34H42ClF2N3O5/c1-43-12-2-3-22-15-23(4-7-31(41)39-10-13-44-14-11-39)32(35)24(16-22)20-40(26-5-6-26)33(42)28-19-38-9-8-34(28)27-18-30(37)29(36)17-25(27)21-45-34/h15-18,26,28,38H,2-14,19-21H2,1H3/t28-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human renin using 9 DNP-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-D,L-Amp as substrate after 3 hrs by Q-FRET assay in presence of... |
Bioorg Med Chem Lett 21: 7399-404 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.013
BindingDB Entry DOI: 10.7270/Q2PC32SW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50359195

(CHEMBL1923132)Show SMILES COCCCc1cc(CCC(=O)N2CCOCC2)c(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@]22OCc3cc(F)c(F)cc23)c1 |r| Show InChI InChI=1S/C34H42ClF2N3O5/c1-43-12-2-3-22-15-23(4-7-31(41)39-10-13-44-14-11-39)32(35)24(16-22)20-40(26-5-6-26)33(42)28-19-38-9-8-34(28)27-18-30(37)29(36)17-25(27)21-45-34/h15-18,26,28,38H,2-14,19-21H2,1H3/t28-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human renin in human plasma using QXL520-Lys-His-Pro-Phe-His-Leu-Val-Ile-His-Lys-(5-FAM) as substrate preincubated for 10 m... |
Bioorg Med Chem Lett 21: 7399-404 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.013
BindingDB Entry DOI: 10.7270/Q2PC32SW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50359195

(CHEMBL1923132)Show SMILES COCCCc1cc(CCC(=O)N2CCOCC2)c(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@]22OCc3cc(F)c(F)cc23)c1 |r| Show InChI InChI=1S/C34H42ClF2N3O5/c1-43-12-2-3-22-15-23(4-7-31(41)39-10-13-44-14-11-39)32(35)24(16-22)20-40(26-5-6-26)33(42)28-19-38-9-8-34(28)27-18-30(37)29(36)17-25(27)21-45-34/h15-18,26,28,38H,2-14,19-21H2,1H3/t28-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Reversible inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 7399-404 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.013
BindingDB Entry DOI: 10.7270/Q2PC32SW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data