 Found 4 hits for monomerid = 50366413
Found 4 hits for monomerid = 50366413 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Xaa-Pro dipeptidase
(Homo sapiens (Human)) | BDBM50366413
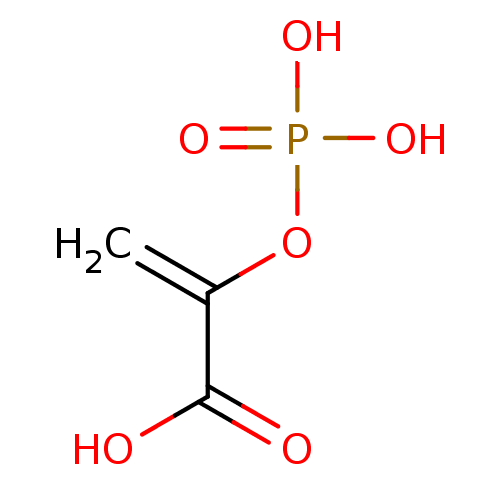
(PHOSPHOENOLPYRUVATE)Show InChI InChI=1S/C3H5O6P/c1-2(3(4)5)9-10(6,7)8/h1H2,(H,4,5)(H2,6,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of prolidase from swine kidney at a pH 6 |
Bioorg Med Chem Lett 5: 627-630 (1995)
Article DOI: 10.1016/0960-894X(95)00085-8
BindingDB Entry DOI: 10.7270/Q2W096DZ |
More data for this
Ligand-Target Pair | |
Xaa-Pro dipeptidase
(Homo sapiens (Human)) | BDBM50366413

(PHOSPHOENOLPYRUVATE)Show InChI InChI=1S/C3H5O6P/c1-2(3(4)5)9-10(6,7)8/h1H2,(H,4,5)(H2,6,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant for inhibition of prolidase in the first phase of biphasic inhibition |
Bioorg Med Chem Lett 5: 627-630 (1995)
Article DOI: 10.1016/0960-894X(95)00085-8
BindingDB Entry DOI: 10.7270/Q2W096DZ |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP] [H477R]
(Rattus norvegicus (Rat)) | BDBM50366413

(PHOSPHOENOLPYRUVATE)Show InChI InChI=1S/C3H5O6P/c1-2(3(4)5)9-10(6,7)8/h1H2,(H,4,5)(H2,6,7,8) | PDB
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
| Assay Description
The inhibition of H477R PEPCK by β-sulfopyruvate (βSP), oxalate, and GMPPCP was conducted in the direction of PEP synthesis (OAA → PE... |
Biochemistry 56: 2106-2115 (2017)
Article DOI: 10.1021/acs.biochem.7b00178
BindingDB Entry DOI: 10.7270/Q2D50KTV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Solute carrier organic anion transporter family member 2A1
(Homo sapiens (Human)) | BDBM50366413

(PHOSPHOENOLPYRUVATE)Show InChI InChI=1S/C3H5O6P/c1-2(3(4)5)9-10(6,7)8/h1H2,(H,4,5)(H2,6,7,8) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.30E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PGE2 uptake in PGT-expressing HeLa cells |
Am J Physiol Renal Physiol 282: 1097-102 (2002)
Article DOI: 10.1152/ajprenal.00151.2001
BindingDB Entry DOI: 10.7270/Q21V5G7C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data