

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50366799 TUBOCURARINE::TUBOCURARINE CHLORIDE
SMILES: COc1cc2CC[N+](C)(C)[C@@H]3Cc4ccc(O)c(Oc5cc6[C@H](Cc7ccc(Oc(c1O)c23)cc7)N(C)CCc6cc5OC)c4
InChI Key: InChIKey=JFJZZMVDLULRGK-URLMMPGGSA-O
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor (Rattus norvegicus (Rat)) | BDBM50366799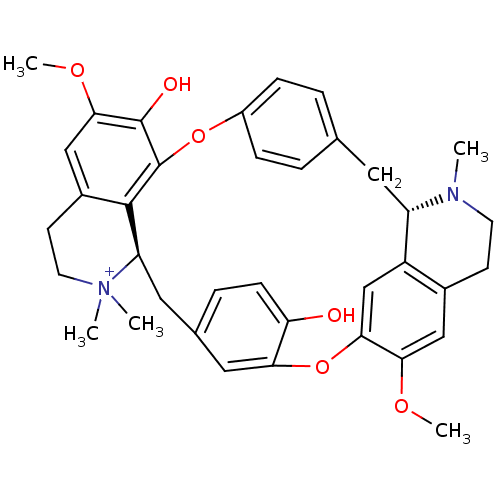 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | 4.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding affinity nicotinic acetylcholine receptor alpha-7 was evaluated by its ability to inhibit [3H]methyllycaconitine ([3H]-MLA) binding to rat br... | Bioorg Med Chem Lett 12: 3067-71 (2002) BindingDB Entry DOI: 10.7270/Q2Q81DNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Small conductance calcium-activated potassium channel protein 3 (Rattus norvegicus) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeuroSearch A/S Curated by ChEMBL | Assay Description Displacement of [I125]apamine from Wistar rat recombinant SK3 channel expressed in HEK293 cells | J Med Chem 51: 7625-34 (2009) Article DOI: 10.1021/jm800809f BindingDB Entry DOI: 10.7270/Q28S4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor protein alpha-4/beta-2 subunit (Rattus norvegicus (Rat)) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | 9.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding affinity for nicotinic acetylcholine receptor alpha4-beta2 was evaluated by its ability to inhibit [3H]NIC binding to rat brain membranes | Bioorg Med Chem Lett 12: 3067-71 (2002) BindingDB Entry DOI: 10.7270/Q2Q81DNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 2 (Homo sapiens (Human)) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 7.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human OCT2-mediated ASP+ uptake expressed in HEK293 cells after 3 mins by fluorescence assay | J Med Chem 56: 781-95 (2013) Article DOI: 10.1021/jm301302s BindingDB Entry DOI: 10.7270/Q2F76DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug and toxin extrusion protein 1 (Homo sapiens (Human)) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human MATE1-mediated ASP+ uptake expressed in HEK293 cells after 1.5 mins by fluorescence assay | J Med Chem 56: 781-95 (2013) Article DOI: 10.1021/jm301302s BindingDB Entry DOI: 10.7270/Q2F76DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Small conductance calcium-activated potassium channel protein 3 (Rattus norvegicus) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a |
NeuroSearch A/S Curated by ChEMBL | Assay Description Inhibition of Wistar rat recombinant SK3 channel expressed in HEK293 cells by whole cell patch clamp technique | J Med Chem 51: 7625-34 (2009) Article DOI: 10.1021/jm800809f BindingDB Entry DOI: 10.7270/Q28S4QT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human BSEP expressed in fall armyworm sf9 cell plasma membrane vesicles assessed as reduction in vesicle-associated [3H]-taurocholate t... | Toxicol Sci 118: 485-500 (2010) Article DOI: 10.1093/toxsci/kfq269 BindingDB Entry DOI: 10.7270/Q26Q20JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 4 (Homo sapiens (Human)) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Canalicular multispecific organic anion transporter 2 (Homo sapiens (Human)) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Canalicular multispecific organic anion transporter 1 (Homo sapiens (Human)) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Small conductance calcium-activated potassium channel (Rattus norvegicus-RAT-Rattus norvegicus (Rat)) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description The SKCa-blocking action was assessed by its ability to inhibit the after hyperpolarization (AHP) in cultured rat sympathetic neurones | J Med Chem 38: 3536-46 (1995) Article DOI: 10.1021/jm00018a013 BindingDB Entry DOI: 10.7270/Q2T156CQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug and toxin extrusion protein 2 (Homo sapiens (Human)) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human MATE2K-mediated ASP+ uptake expressed in HEK293 cells after 1.5 mins by fluorescence assay | J Med Chem 56: 781-95 (2013) Article DOI: 10.1021/jm301302s BindingDB Entry DOI: 10.7270/Q2F76DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Inhibition of HIV1 RT | J Nat Prod 54: 143-54 Article DOI: 10.1021/np50073a012 BindingDB Entry DOI: 10.7270/Q2NK3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor Alpha-4/Beta-2 (Homo sapiens (Human)) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Antagonist activity at human alpha4beta2 nAChR expressed in HEK-tsA201 cells assessed as inhibition of epibatidine-induced intracellular calcium leve... | Bioorg Med Chem 21: 4730-43 (2013) Article DOI: 10.1016/j.bmc.2013.03.082 BindingDB Entry DOI: 10.7270/Q2V12673 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor; alpha3/beta4 (Homo sapiens (Human)) | BDBM50366799 (TUBOCURARINE | TUBOCURARINE CHLORIDE) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University Curated by ChEMBL | Assay Description Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion | Bioorg Med Chem Lett 14: 3739-42 (2004) Article DOI: 10.1016/j.bmcl.2004.05.001 BindingDB Entry DOI: 10.7270/Q28G8M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||