

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: NC(=O)[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12
InChI Key: InChIKey=BLMHAOGGJQDPLX-TXDDJGQJSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-oxoglutarate receptor 1 (Rattus norvegicus) | BDBM50367376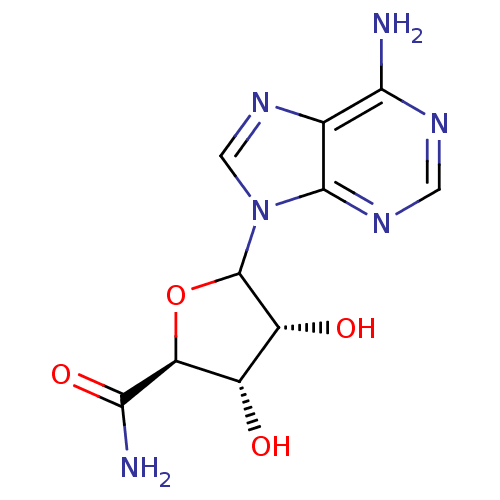 (CHEMBL605866) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Binding activity of P3 purinoceptor-like protein (P3LP) using radioligand 40 nM [3H]NECA from rat brain membranes | J Med Chem 44: 208-14 (2001) BindingDB Entry DOI: 10.7270/Q2H41S5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50367376 (CHEMBL605866) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity for A1-adenosine receptor by the displacement of specific [3H]-PIA binding in rat brain was determined | J Med Chem 37: 636-46 (1994) BindingDB Entry DOI: 10.7270/Q2057GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50367376 (CHEMBL605866) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity for recombinant rat A3-adenosine receptor by the displacement of specific [125I]APNEA or [125I]-N6-(4-amino-3-iodobenzyl)-adenosine-... | J Med Chem 37: 636-46 (1994) BindingDB Entry DOI: 10.7270/Q2057GJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50367376 (CHEMBL605866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. | J Med Chem 39: 4162-6 (1996) Article DOI: 10.1021/jm960313y BindingDB Entry DOI: 10.7270/Q2DN45QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50367376 (CHEMBL605866) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Potency against rat brain adenosine A1 receptor | J Med Chem 29: 1683-9 (1986) BindingDB Entry DOI: 10.7270/Q2Z89D0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50367376 (CHEMBL605866) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Potency against human platelet A2 adenosine receptor | J Med Chem 29: 1683-9 (1986) BindingDB Entry DOI: 10.7270/Q2Z89D0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50367376 (CHEMBL605866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Potency against PC12 cell A2 adenosine receptor by adenylate cyclase activation | J Med Chem 29: 1683-9 (1986) BindingDB Entry DOI: 10.7270/Q2Z89D0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||