 Found 7 hits for monomerid = 50373123
Found 7 hits for monomerid = 50373123 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50373123
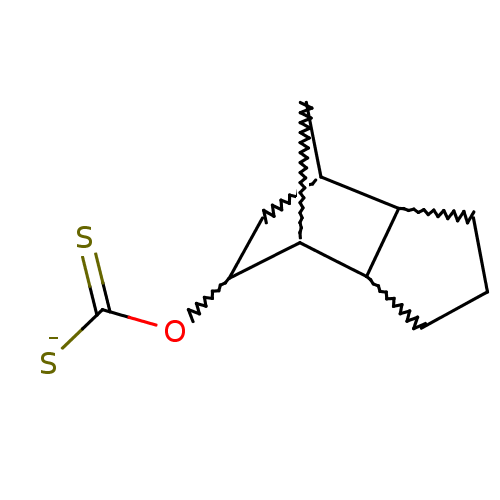
(CHEMBL404954)Show SMILES [S-]C(=S)OC1CC2CC1C1CCCC21 |w:9.10,13.13,6.5,4.3,8.7,TEB:10:9:7:4.5,12:13:7:4.5,3:4:7:13.9| Show InChI InChI=1S/C11H16OS2/c13-11(14)12-10-5-6-4-9(10)8-3-1-2-7(6)8/h6-10H,1-5H2,(H,13,14)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA2 cytosolic isoform preincubated for 15 mins by stopped-flow CO2 hydration assay |
J Med Chem 56: 4691-700 (2013)
Article DOI: 10.1021/jm400414j
BindingDB Entry DOI: 10.7270/Q2M61MSD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50373123

(CHEMBL404954)Show SMILES [S-]C(=S)OC1CC2CC1C1CCCC21 |w:9.10,13.13,6.5,4.3,8.7,TEB:10:9:7:4.5,12:13:7:4.5,3:4:7:13.9| Show InChI InChI=1S/C11H16OS2/c13-11(14)12-10-5-6-4-9(10)8-3-1-2-7(6)8/h6-10H,1-5H2,(H,13,14)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA1 cytosolic isoform preincubated for 15 mins by stopped-flow CO2 hydration assay |
J Med Chem 56: 4691-700 (2013)
Article DOI: 10.1021/jm400414j
BindingDB Entry DOI: 10.7270/Q2M61MSD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50373123

(CHEMBL404954)Show SMILES [S-]C(=S)OC1CC2CC1C1CCCC21 |w:9.10,13.13,6.5,4.3,8.7,TEB:10:9:7:4.5,12:13:7:4.5,3:4:7:13.9| Show InChI InChI=1S/C11H16OS2/c13-11(14)12-10-5-6-4-9(10)8-3-1-2-7(6)8/h6-10H,1-5H2,(H,13,14)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 643 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA9 transmembrane isoform preincubated for 15 mins by stopped-flow CO2 hydration assay |
J Med Chem 56: 4691-700 (2013)
Article DOI: 10.1021/jm400414j
BindingDB Entry DOI: 10.7270/Q2M61MSD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50373123

(CHEMBL404954)Show SMILES [S-]C(=S)OC1CC2CC1C1CCCC21 |w:9.10,13.13,6.5,4.3,8.7,TEB:10:9:7:4.5,12:13:7:4.5,3:4:7:13.9| Show InChI InChI=1S/C11H16OS2/c13-11(14)12-10-5-6-4-9(10)8-3-1-2-7(6)8/h6-10H,1-5H2,(H,13,14)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 835 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA12 transmembrane isoform preincubated for 15 mins by stopped-flow CO2 hydration assay |
J Med Chem 56: 4691-700 (2013)
Article DOI: 10.1021/jm400414j
BindingDB Entry DOI: 10.7270/Q2M61MSD |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50373123

(CHEMBL404954)Show SMILES [S-]C(=S)OC1CC2CC1C1CCCC21 |w:9.10,13.13,6.5,4.3,8.7,TEB:10:9:7:4.5,12:13:7:4.5,3:4:7:13.9| Show InChI InChI=1S/C11H16OS2/c13-11(14)12-10-5-6-4-9(10)8-3-1-2-7(6)8/h6-10H,1-5H2,(H,13,14)/p-1 | PDB
MMDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of cruzain in presence of 0.01% Triton X-100 |
J Med Chem 51: 2502-11 (2008)
Article DOI: 10.1021/jm701500e
BindingDB Entry DOI: 10.7270/Q23R0TQG |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50373123

(CHEMBL404954)Show SMILES [S-]C(=S)OC1CC2CC1C1CCCC21 |w:9.10,13.13,6.5,4.3,8.7,TEB:10:9:7:4.5,12:13:7:4.5,3:4:7:13.9| Show InChI InChI=1S/C11H16OS2/c13-11(14)12-10-5-6-4-9(10)8-3-1-2-7(6)8/h6-10H,1-5H2,(H,13,14)/p-1 | PDB
MMDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of cruzain |
J Med Chem 51: 2502-11 (2008)
Article DOI: 10.1021/jm701500e
BindingDB Entry DOI: 10.7270/Q23R0TQG |
More data for this
Ligand-Target Pair | |
Phosphatidylcholine:ceramide cholinephosphotransferase 2
(Homo sapiens (Human)) | BDBM50373123

(CHEMBL404954)Show SMILES [S-]C(=S)OC1CC2CC1C1CCCC21 |w:9.10,13.13,6.5,4.3,8.7,TEB:10:9:7:4.5,12:13:7:4.5,3:4:7:13.9| Show InChI InChI=1S/C11H16OS2/c13-11(14)12-10-5-6-4-9(10)8-3-1-2-7(6)8/h6-10H,1-5H2,(H,13,14)/p-1 | Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of SMS2 (unknown origin) expressed in H5 insect cells using C6-NBD-Cer as substrate after 1 hr |
Eur J Med Chem 73: 1-7 (2014)
Article DOI: 10.1016/j.ejmech.2013.12.002
BindingDB Entry DOI: 10.7270/Q2805430 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data