

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50373877 Betaxin::CA inhibitor, 3::THIAMINE (VIT B1)::ThOH::Thiamine::Vitamin B 1
SMILES: Cc1c(CCO)sc[n+]1Cc1cnc(C)nc1N
InChI Key: InChIKey=JZRWCGZRTZMZEH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 6 (CA-VI) (Homo sapiens (Human)) | BDBM50373877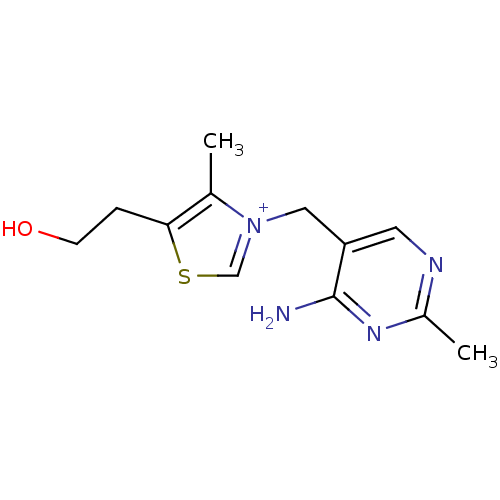 (Betaxin | CA inhibitor, 3 | THIAMINE (VIT B1) | Th...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 62 | -9.83 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Kirklareli University | Assay Description Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 10 mM Tris-HCl (pH 7.5) as bu... | J Enzyme Inhib Med Chem 28: 316-9 (2013) Article DOI: 10.3109/14756366.2011.637200 BindingDB Entry DOI: 10.7270/Q208647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50373877 (Betaxin | CA inhibitor, 3 | THIAMINE (VIT B1) | Th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 85 | -9.64 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Kirklareli University | Assay Description Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 10 mM Tris-HCl (pH 7.5) as bu... | J Enzyme Inhib Med Chem 28: 316-9 (2013) Article DOI: 10.3109/14756366.2011.637200 BindingDB Entry DOI: 10.7270/Q208647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50373877 (Betaxin | CA inhibitor, 3 | THIAMINE (VIT B1) | Th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 380 | -8.75 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Kirklareli University | Assay Description Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 10 mM Tris-HCl (pH 7.5) as bu... | J Enzyme Inhib Med Chem 28: 316-9 (2013) Article DOI: 10.3109/14756366.2011.637200 BindingDB Entry DOI: 10.7270/Q208647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50373877 (Betaxin | CA inhibitor, 3 | THIAMINE (VIT B1) | Th...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.25 | n/a |
TBA Curated by ChEMBL | Assay Description Noncompetitive inhibition of electric eel AChE using acetylthiocholine chloride as substrate at pH 8.25 by Michaelis-Menten plot analysis | J Med Chem 20: 161-4 (1977) BindingDB Entry DOI: 10.7270/Q2JD4ZB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50373877 (Betaxin | CA inhibitor, 3 | THIAMINE (VIT B1) | Th...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.25 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine chloride as substrate at pH 8.25 by Michaelis-Menten plot analysis | J Med Chem 20: 161-4 (1977) BindingDB Entry DOI: 10.7270/Q2JD4ZB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50373877 (Betaxin | CA inhibitor, 3 | THIAMINE (VIT B1) | Th...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine chloride as substrate at pH 7 by Michaelis-Menten plot analysis | J Med Chem 20: 161-4 (1977) BindingDB Entry DOI: 10.7270/Q2JD4ZB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50373877 (Betaxin | CA inhibitor, 3 | THIAMINE (VIT B1) | Th...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Noncompetitive inhibition of electric eel AChE using acetylthiocholine chloride as substrate at pH 7 by Michaelis-Menten plot analysis | J Med Chem 20: 161-4 (1977) BindingDB Entry DOI: 10.7270/Q2JD4ZB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenaline alpha2 (RAT) | BDBM50373877 (Betaxin | CA inhibitor, 3 | THIAMINE (VIT B1) | Th...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 6.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Hospital Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of TEA uptake in OCT2-expressing MDCK cells | Pharm Res 18: 1528-34 (2001) BindingDB Entry DOI: 10.7270/Q28G8N0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenaline alpha1 (RAT) | BDBM50373877 (Betaxin | CA inhibitor, 3 | THIAMINE (VIT B1) | Th...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 7.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Hospital Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of TEA uptake in OCT1-expressing MDCK cells | Pharm Res 18: 1528-34 (2001) BindingDB Entry DOI: 10.7270/Q28G8N0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50373877 (Betaxin | CA inhibitor, 3 | THIAMINE (VIT B1) | Th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Homo sapiens (human) Hsp90 alpha ATPase activity assessed as inorganic phosphate release by malachite green colorimetric me... | Citation and Details Article DOI: 10.1007/s00044-011-9557-9 BindingDB Entry DOI: 10.7270/Q2V127QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transketolase (Homo sapiens (Human)) | BDBM50373877 (Betaxin | CA inhibitor, 3 | THIAMINE (VIT B1) | Th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 6.80E+6 | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. Curated by ChEMBL | Assay Description Inhibition of apo-transketolase | Bioorg Med Chem Lett 18: 509-12 (2008) Article DOI: 10.1016/j.bmcl.2007.11.098 BindingDB Entry DOI: 10.7270/Q2WW7JJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic Alpha (Homo sapiens (Human)) | BDBM50373877 (Betaxin | CA inhibitor, 3 | THIAMINE (VIT B1) | Th...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.35E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human OCT1 expressed in HEK293 cells assessed as decrease in uptake of MPP+ after 1 min | J Med Chem 60: 2685-2696 (2017) Article DOI: 10.1021/acs.jmedchem.6b01317 BindingDB Entry DOI: 10.7270/Q2NV9MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiamine-binding periplasmic protein (Escherichia coli (strain K12)) | BDBM50373877 (Betaxin | CA inhibitor, 3 | THIAMINE (VIT B1) | Th...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a |
Cornell University | Assay Description the kd for ThOH, ThMP and ThDP were determined using titration experiments in which the change in the intrinsic protein fluorescence was monitored as... | Biochemistry 47: 1346-57 (2008) Article DOI: 10.1021/bi7018282 BindingDB Entry DOI: 10.7270/Q28C9TVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||