

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50380203 CHEMBL253152
SMILES: [#6]-[#8]-c1cc(ccc1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2c(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])cc2-[#8]-1
InChI Key: InChIKey=PMKNILHJZILHLN-AGBQBWRISA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50380203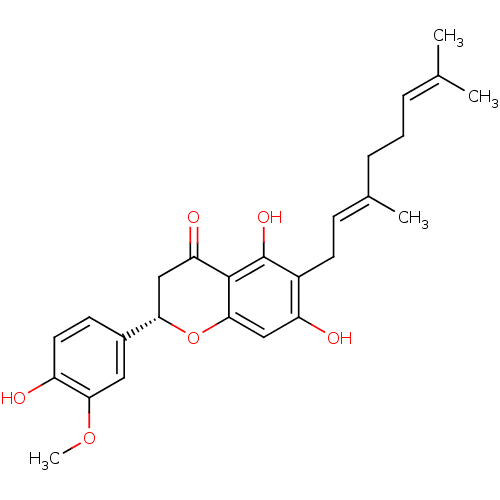 (CHEMBL253152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Noncompetitive inhibition of human neutrophil elastase using varying levels of MeOSuc-AAPV-pNA as substrate measured after 30 mins by double-reciproc... | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BChE) (Equus caballus (Horse)) | BDBM50380203 (CHEMBL253152) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of equine BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk double-reciprocal-plot and dixon plot analysis | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50380203 (CHEMBL253152) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk double-reciprocal-plot and dixon plot ... | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50380203 (CHEMBL253152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-pNA as substrate measured after 30 mins by spectrometric method | J Nat Prod 80: 2659-2665 (2017) Article DOI: 10.1021/acs.jnatprod.7b00325 BindingDB Entry DOI: 10.7270/Q2RX9FK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50380203 (CHEMBL253152) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE assessed as acetylthiocholine iodide hydrolysis after 10 mins preincubation by spectrophotometry | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BChE) (Equus caballus (Horse)) | BDBM50380203 (CHEMBL253152) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of equine BChE assessed as butyrylthiocholine iodide hydrolysis after 10 mins preincubation by spectrophotometry | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||