 Found 14 hits for monomerid = 50382163
Found 14 hits for monomerid = 50382163 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aldo-keto reductase family 1 member C4 (AK1C4)
(Homo sapiens (Human)) | BDBM50382163
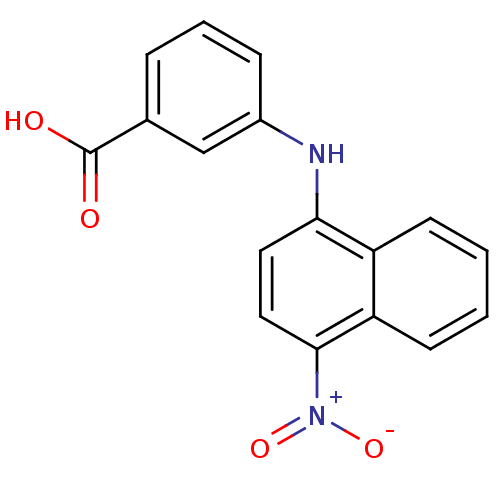
(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C4 assessed as enzyme catalyzed oxidation of S-tetralol by fluorimetric assay |
Bioorg Med Chem Lett 22: 3492-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.085
BindingDB Entry DOI: 10.7270/Q24M95JQ |
More data for this
Ligand-Target Pair | |
17-beta-Hydroxysteroid Dehydrogenase 5 (17-beta-HSD5, AKR1C3)
(Homo sapiens (Human)) | BDBM50382163

(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 assessed as enzyme catalyzed oxidation of S-tetralol by fluorimetric assay |
Bioorg Med Chem Lett 22: 3492-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.085
BindingDB Entry DOI: 10.7270/Q24M95JQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen Receptor
(Homo sapiens (Human)) | BDBM50382163

(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
| Assay Description
Antagonist activity at androgen receptor expressed in HeLa-AR3A-PSA-(ARE)4-Luc13 cells assessed as rightward shift of DHT-induced response incubated ... |
Bioorg Med Chem Lett 22: 3492-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.085
BindingDB Entry DOI: 10.7270/Q24M95JQ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50382163

(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C1 assessed as enzyme catalyzed oxidation of S-tetralol by fluorimetric assay |
Bioorg Med Chem Lett 22: 3492-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.085
BindingDB Entry DOI: 10.7270/Q24M95JQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50382163

(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C2 assessed as enzyme catalyzed oxidation of S-tetralol by fluorimetric assay |
Bioorg Med Chem Lett 22: 3492-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.085
BindingDB Entry DOI: 10.7270/Q24M95JQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclooxygenase-1 (COX-1)
(Homo sapiens (Human)) | BDBM50382163

(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of recombinant COX1 |
Bioorg Med Chem Lett 22: 3492-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.085
BindingDB Entry DOI: 10.7270/Q24M95JQ |
More data for this
Ligand-Target Pair | |
Androgen receptor/Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50382163

(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor/Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50382163

(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay |
J Med Chem 63: 10396-10411 (2020)
|
More data for this
Ligand-Target Pair | |
Androgen receptor/Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50382163

(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C4 (AK1C4)
(Homo sapiens (Human)) | BDBM50382163

(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 3.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50382163

(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto-reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50382163

(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50382163

(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 4.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclooxygenase
(Homo sapiens (Human)) | BDBM50382163

(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of recombinant COX2 |
Bioorg Med Chem Lett 22: 3492-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.085
BindingDB Entry DOI: 10.7270/Q24M95JQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data