

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50382337 CHEMBL2024335
SMILES: CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSC(CC(=O)c1ccc(Cl)cc1)C(O)=O
InChI Key: InChIKey=GTXSZCCMMWMXRE-DVXAHSAASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1,4-Dihydroxy-2-naphthoyl-CoA synthase (Mycobacterium tuberculosis) | BDBM50382337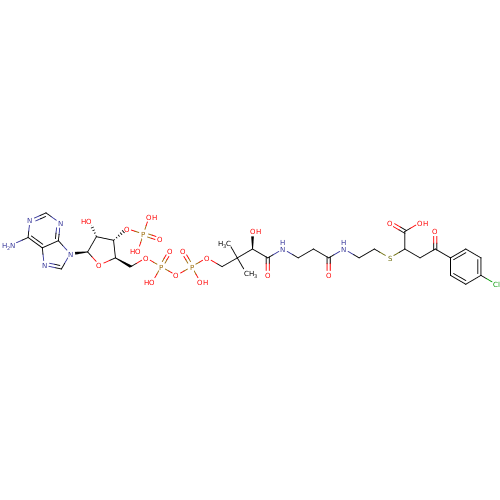 (CHEMBL2024335) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MenB expressed in Escherichia coli BL21 (DE3) by Lineweaver-Burk plot analysis | ACS Med Chem Lett 2: 818-823 (2011) Article DOI: 10.1021/ml200141e BindingDB Entry DOI: 10.7270/Q2B56KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,4-Dihydroxy-2-naphthoyl-CoA synthase (Mycobacterium tuberculosis) | BDBM50382337 (CHEMBL2024335) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Non-competitive inhibition of Mycobacterium tuberculosis MenB expressed in Escherichia coli BL21 (DE3) by Lineweaver-Burk plot analysis | ACS Med Chem Lett 2: 818-823 (2011) Article DOI: 10.1021/ml200141e BindingDB Entry DOI: 10.7270/Q2B56KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,4-Dihydroxy-2-naphthoyl-CoA synthase (Mycobacterium tuberculosis) | BDBM50382337 (CHEMBL2024335) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MenB expressed in Escherichia coli BL21 (DE3) assessed as formation of DHNA-CoA from OSB-CoA by enzyme coupl... | ACS Med Chem Lett 2: 818-823 (2011) Article DOI: 10.1021/ml200141e BindingDB Entry DOI: 10.7270/Q2B56KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||