

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50383374 CHEMBL2030555::CHEMBL2069960::US10544104, Compound 30::US9765037, Compound 30
SMILES: Nc1ncnc2n(CC3CCNCC3)nc(-c3cnc4ccccc4c3)c12
InChI Key: InChIKey=JSONRPFPVGYCOB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50383374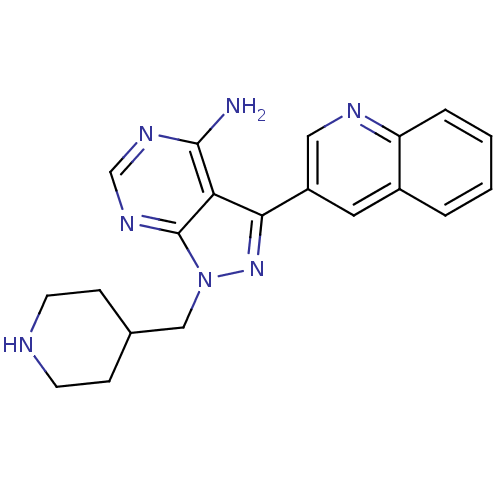 (CHEMBL2030555 | CHEMBL2069960 | US10544104, Compou...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human ABL by radiometric assay | J Med Chem 55: 2803-10 (2012) Article DOI: 10.1021/jm201725v BindingDB Entry DOI: 10.7270/Q2ST7QVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50383374 (CHEMBL2030555 | CHEMBL2069960 | US10544104, Compou...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human SRC by radiometric assay | J Med Chem 55: 2803-10 (2012) Article DOI: 10.1021/jm201725v BindingDB Entry DOI: 10.7270/Q2ST7QVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-dependent protein kinase 1 (CDPK1) (Cryptosporidium parvum) | BDBM50383374 (CHEMBL2030555 | CHEMBL2069960 | US10544104, Compou...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 83.7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Through its Center for Commercialization US Patent | Assay Description Two types of enzyme assays were developed to follow TgCDPK1 activity, a radiometric scintillation proximity assay measured the labeled γ-phospha... | US Patent US9765037 (2017) BindingDB Entry DOI: 10.7270/Q2B56MVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50383374 (CHEMBL2030555 | CHEMBL2069960 | US10544104, Compou...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF WASHINGTON THROUGH ITS CENTER FOR CO US Patent | Assay Description Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti... | US Patent US10544104 (2020) BindingDB Entry DOI: 10.7270/Q2D79DSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50383374 (CHEMBL2030555 | CHEMBL2069960 | US10544104, Compou...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Through its Center for Commercialization US Patent | Assay Description Inhibition of human tyrosine kinases. | US Patent US9765037 (2017) BindingDB Entry DOI: 10.7270/Q2B56MVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calmodulin-domain protein kinase 1 (Toxoplasma gondii) | BDBM50383374 (CHEMBL2030555 | CHEMBL2069960 | US10544104, Compou...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF WASHINGTON THROUGH ITS CENTER FOR CO US Patent | Assay Description Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti... | US Patent US10544104 (2020) BindingDB Entry DOI: 10.7270/Q2D79DSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calmodulin-domain protein kinase 1, putative (Cryptosporidium parvum (strain Iowa II)) | BDBM50383374 (CHEMBL2030555 | CHEMBL2069960 | US10544104, Compou...) | MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 83.7 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF WASHINGTON THROUGH ITS CENTER FOR CO US Patent | Assay Description Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti... | US Patent US10544104 (2020) BindingDB Entry DOI: 10.7270/Q2D79DSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calmodulin/Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50383374 (CHEMBL2030555 | CHEMBL2069960 | US10544104, Compou...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF WASHINGTON THROUGH ITS CENTER FOR CO US Patent | Assay Description Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti... | US Patent US10544104 (2020) BindingDB Entry DOI: 10.7270/Q2D79DSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calmodulin-domain protein kinase 1 (CDPK1) (Toxoplasma gondii) | BDBM50383374 (CHEMBL2030555 | CHEMBL2069960 | US10544104, Compou...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Through its Center for Commercialization US Patent | Assay Description Most known kinase inhibitors bind in the ATP-binding pocket of the active site19,20. These inhibitors exploit many of the same hydrophobic contacts a... | US Patent US9765037 (2017) BindingDB Entry DOI: 10.7270/Q2B56MVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50383374 (CHEMBL2030555 | CHEMBL2069960 | US10544104, Compou...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human SRC using Ac-EIYGEFKKK-OH as substrate after 60 mins by phosphorimaging method | J Med Chem 55: 2416-26 (2012) Article DOI: 10.1021/jm201713h BindingDB Entry DOI: 10.7270/Q2P2706V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50383374 (CHEMBL2030555 | CHEMBL2069960 | US10544104, Compou...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Through its Center for Commercialization US Patent | Assay Description Inhibition of human tyrosine kinases. | US Patent US9765037 (2017) BindingDB Entry DOI: 10.7270/Q2B56MVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||