

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50384429 CHEMBL2031492::US9896452, Example 137
SMILES: C[C@H]1CCCN([C@@H]1CNC(=O)c1cccc2cccnc12)C(=O)c1nc(C)sc1-c1ccccc1
InChI Key: InChIKey=SRKVUFVTSRBFFN-FDDCHVKYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50384429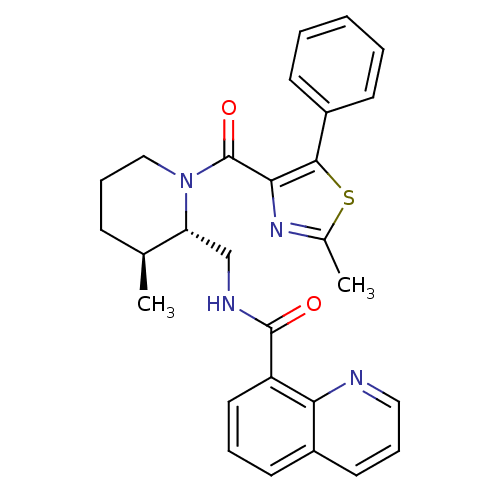 (CHEMBL2031492 | US9896452, Example 137) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Florida Curated by ChEMBL | Assay Description Antagonist activity at OX2 receptor expressed in CHO cells assessed as inhibition of OXA-stimulated intracellular calcium mobilization after 30 mins ... | Bioorg Med Chem Lett 22: 3890-4 (2012) Article DOI: 10.1016/j.bmcl.2012.04.122 BindingDB Entry DOI: 10.7270/Q2M046GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50384429 (CHEMBL2031492 | US9896452, Example 137) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 1 (Homo sapiens (Human)) | BDBM50384429 (CHEMBL2031492 | US9896452, Example 137) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description Measurement of [Ca2+]i using a FLIPR: CHO-OX1 or CHO-OX2 cells are seeded into black-walled clear-base 384-well plates (Costar) at a density of 20,00... | J Med Chem 48: 2906-15 (2005) BindingDB Entry DOI: 10.7270/Q22J6F5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 1 (Homo sapiens (Human)) | BDBM50384429 (CHEMBL2031492 | US9896452, Example 137) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Florida Curated by ChEMBL | Assay Description Antagonist activity at OX1 receptor expressed in CHO cells assessed as inhibition of OXA-stimulated intracellular calcium mobilization after 30 mins ... | Bioorg Med Chem Lett 22: 3890-4 (2012) Article DOI: 10.1016/j.bmcl.2012.04.122 BindingDB Entry DOI: 10.7270/Q2M046GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||