

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50384466 CHEMBL2036212
SMILES: CCCCC(=O)N(Cc1cccc(OC(F)(F)F)c1)c1cc(F)cc(c1)-c1nnn[nH]1
InChI Key: InChIKey=UAHVGQVTFGOJQH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50384466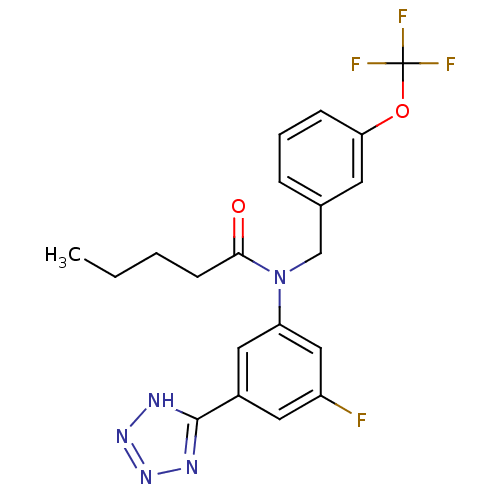 (CHEMBL2036212) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3HPGD2 from human CRTH2 receptor expressed in CHO cell membrane after 90 mins by scintillation proximity assay | ACS Med Chem Lett 2: 938-942 (2011) Article DOI: 10.1021/ml200223s BindingDB Entry DOI: 10.7270/Q2Z89DGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50384466 (CHEMBL2036212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry | ACS Med Chem Lett 2: 938-942 (2011) Article DOI: 10.1021/ml200223s BindingDB Entry DOI: 10.7270/Q2Z89DGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50384466 (CHEMBL2036212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry | ACS Med Chem Lett 2: 938-942 (2011) Article DOI: 10.1021/ml200223s BindingDB Entry DOI: 10.7270/Q2Z89DGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50384466 (CHEMBL2036212) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry | ACS Med Chem Lett 2: 938-942 (2011) Article DOI: 10.1021/ml200223s BindingDB Entry DOI: 10.7270/Q2Z89DGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50384466 (CHEMBL2036212) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2B6 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry | ACS Med Chem Lett 2: 938-942 (2011) Article DOI: 10.1021/ml200223s BindingDB Entry DOI: 10.7270/Q2Z89DGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||