 Found 8 hits for monomerid = 50384600
Found 8 hits for monomerid = 50384600 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50384600
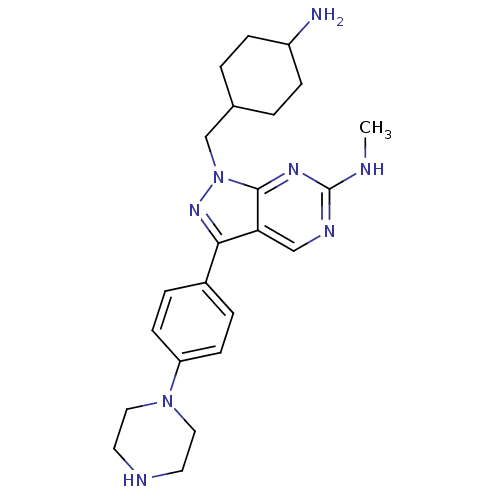
(CHEMBL2036792 | US9744172, Compound UNC00000563A)Show SMILES CNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |(53.41,-28.01,;54.75,-28.78,;56.08,-28.01,;56.08,-26.46,;57.41,-25.69,;58.74,-26.46,;60.22,-25.98,;61.13,-27.23,;60.22,-28.49,;60.7,-29.95,;59.67,-31.09,;58.16,-30.76,;57.13,-31.9,;57.6,-33.37,;56.56,-34.51,;59.11,-33.69,;60.14,-32.55,;58.75,-28.01,;57.41,-28.78,;60.69,-24.51,;62.2,-24.2,;62.68,-22.74,;61.65,-21.59,;60.13,-21.92,;59.67,-23.38,;62.12,-20.13,;63.62,-19.81,;64.1,-18.35,;63.07,-17.2,;61.56,-17.52,;61.08,-18.99,)| Show InChI InChI=1S/C23H32N8/c1-25-23-27-14-20-21(17-4-8-19(9-5-17)30-12-10-26-11-13-30)29-31(22(20)28-23)15-16-2-6-18(24)7-3-16/h4-5,8-9,14,16,18,26H,2-3,6-7,10-13,15,24H2,1H3,(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Axl using KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assay |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50384600

(CHEMBL2036792 | US9744172, Compound UNC00000563A)Show SMILES CNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |(53.41,-28.01,;54.75,-28.78,;56.08,-28.01,;56.08,-26.46,;57.41,-25.69,;58.74,-26.46,;60.22,-25.98,;61.13,-27.23,;60.22,-28.49,;60.7,-29.95,;59.67,-31.09,;58.16,-30.76,;57.13,-31.9,;57.6,-33.37,;56.56,-34.51,;59.11,-33.69,;60.14,-32.55,;58.75,-28.01,;57.41,-28.78,;60.69,-24.51,;62.2,-24.2,;62.68,-22.74,;61.65,-21.59,;60.13,-21.92,;59.67,-23.38,;62.12,-20.13,;63.62,-19.81,;64.1,-18.35,;63.07,-17.2,;61.56,-17.52,;61.08,-18.99,)| Show InChI InChI=1S/C23H32N8/c1-25-23-27-14-20-21(17-4-8-19(9-5-17)30-12-10-26-11-13-30)29-31(22(20)28-23)15-16-2-6-18(24)7-3-16/h4-5,8-9,14,16,18,26H,2-3,6-7,10-13,15,24H2,1H3,(H,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Tyro3 using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assay |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384600

(CHEMBL2036792 | US9744172, Compound UNC00000563A)Show SMILES CNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |(53.41,-28.01,;54.75,-28.78,;56.08,-28.01,;56.08,-26.46,;57.41,-25.69,;58.74,-26.46,;60.22,-25.98,;61.13,-27.23,;60.22,-28.49,;60.7,-29.95,;59.67,-31.09,;58.16,-30.76,;57.13,-31.9,;57.6,-33.37,;56.56,-34.51,;59.11,-33.69,;60.14,-32.55,;58.75,-28.01,;57.41,-28.78,;60.69,-24.51,;62.2,-24.2,;62.68,-22.74,;61.65,-21.59,;60.13,-21.92,;59.67,-23.38,;62.12,-20.13,;63.62,-19.81,;64.1,-18.35,;63.07,-17.2,;61.56,-17.52,;61.08,-18.99,)| Show InChI InChI=1S/C23H32N8/c1-25-23-27-14-20-21(17-4-8-19(9-5-17)30-12-10-26-11-13-30)29-31(22(20)28-23)15-16-2-6-18(24)7-3-16/h4-5,8-9,14,16,18,26H,2-3,6-7,10-13,15,24H2,1H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50384600

(CHEMBL2036792 | US9744172, Compound UNC00000563A)Show SMILES CNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |(53.41,-28.01,;54.75,-28.78,;56.08,-28.01,;56.08,-26.46,;57.41,-25.69,;58.74,-26.46,;60.22,-25.98,;61.13,-27.23,;60.22,-28.49,;60.7,-29.95,;59.67,-31.09,;58.16,-30.76,;57.13,-31.9,;57.6,-33.37,;56.56,-34.51,;59.11,-33.69,;60.14,-32.55,;58.75,-28.01,;57.41,-28.78,;60.69,-24.51,;62.2,-24.2,;62.68,-22.74,;61.65,-21.59,;60.13,-21.92,;59.67,-23.38,;62.12,-20.13,;63.62,-19.81,;64.1,-18.35,;63.07,-17.2,;61.56,-17.52,;61.08,-18.99,)| Show InChI InChI=1S/C23H32N8/c1-25-23-27-14-20-21(17-4-8-19(9-5-17)30-12-10-26-11-13-30)29-31(22(20)28-23)15-16-2-6-18(24)7-3-16/h4-5,8-9,14,16,18,26H,2-3,6-7,10-13,15,24H2,1H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 62: 1180-1202 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01218 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384600

(CHEMBL2036792 | US9744172, Compound UNC00000563A)Show SMILES CNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |(53.41,-28.01,;54.75,-28.78,;56.08,-28.01,;56.08,-26.46,;57.41,-25.69,;58.74,-26.46,;60.22,-25.98,;61.13,-27.23,;60.22,-28.49,;60.7,-29.95,;59.67,-31.09,;58.16,-30.76,;57.13,-31.9,;57.6,-33.37,;56.56,-34.51,;59.11,-33.69,;60.14,-32.55,;58.75,-28.01,;57.41,-28.78,;60.69,-24.51,;62.2,-24.2,;62.68,-22.74,;61.65,-21.59,;60.13,-21.92,;59.67,-23.38,;62.12,-20.13,;63.62,-19.81,;64.1,-18.35,;63.07,-17.2,;61.56,-17.52,;61.08,-18.99,)| Show InChI InChI=1S/C23H32N8/c1-25-23-27-14-20-21(17-4-8-19(9-5-17)30-12-10-26-11-13-30)29-31(22(20)28-23)15-16-2-6-18(24)7-3-16/h4-5,8-9,14,16,18,26H,2-3,6-7,10-13,15,24H2,1H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of MER (unknown origin) |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50384600

(CHEMBL2036792 | US9744172, Compound UNC00000563A)Show SMILES CNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |(53.41,-28.01,;54.75,-28.78,;56.08,-28.01,;56.08,-26.46,;57.41,-25.69,;58.74,-26.46,;60.22,-25.98,;61.13,-27.23,;60.22,-28.49,;60.7,-29.95,;59.67,-31.09,;58.16,-30.76,;57.13,-31.9,;57.6,-33.37,;56.56,-34.51,;59.11,-33.69,;60.14,-32.55,;58.75,-28.01,;57.41,-28.78,;60.69,-24.51,;62.2,-24.2,;62.68,-22.74,;61.65,-21.59,;60.13,-21.92,;59.67,-23.38,;62.12,-20.13,;63.62,-19.81,;64.1,-18.35,;63.07,-17.2,;61.56,-17.52,;61.08,-18.99,)| Show InChI InChI=1S/C23H32N8/c1-25-23-27-14-20-21(17-4-8-19(9-5-17)30-12-10-26-11-13-30)29-31(22(20)28-23)15-16-2-6-18(24)7-3-16/h4-5,8-9,14,16,18,26H,2-3,6-7,10-13,15,24H2,1H3,(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh
Curated by ChEMBL
| Assay Description
Inhibition of AXL (unknown origin) |
J Med Chem 61: 2104-2110 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01605
BindingDB Entry DOI: 10.7270/Q2D50QKC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50384600

(CHEMBL2036792 | US9744172, Compound UNC00000563A)Show SMILES CNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |(53.41,-28.01,;54.75,-28.78,;56.08,-28.01,;56.08,-26.46,;57.41,-25.69,;58.74,-26.46,;60.22,-25.98,;61.13,-27.23,;60.22,-28.49,;60.7,-29.95,;59.67,-31.09,;58.16,-30.76,;57.13,-31.9,;57.6,-33.37,;56.56,-34.51,;59.11,-33.69,;60.14,-32.55,;58.75,-28.01,;57.41,-28.78,;60.69,-24.51,;62.2,-24.2,;62.68,-22.74,;61.65,-21.59,;60.13,-21.92,;59.67,-23.38,;62.12,-20.13,;63.62,-19.81,;64.1,-18.35,;63.07,-17.2,;61.56,-17.52,;61.08,-18.99,)| Show InChI InChI=1S/C23H32N8/c1-25-23-27-14-20-21(17-4-8-19(9-5-17)30-12-10-26-11-13-30)29-31(22(20)28-23)15-16-2-6-18(24)7-3-16/h4-5,8-9,14,16,18,26H,2-3,6-7,10-13,15,24H2,1H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 (unknown origin) |
J Med Chem 62: 1180-1202 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01218 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384600

(CHEMBL2036792 | US9744172, Compound UNC00000563A)Show SMILES CNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |(53.41,-28.01,;54.75,-28.78,;56.08,-28.01,;56.08,-26.46,;57.41,-25.69,;58.74,-26.46,;60.22,-25.98,;61.13,-27.23,;60.22,-28.49,;60.7,-29.95,;59.67,-31.09,;58.16,-30.76,;57.13,-31.9,;57.6,-33.37,;56.56,-34.51,;59.11,-33.69,;60.14,-32.55,;58.75,-28.01,;57.41,-28.78,;60.69,-24.51,;62.2,-24.2,;62.68,-22.74,;61.65,-21.59,;60.13,-21.92,;59.67,-23.38,;62.12,-20.13,;63.62,-19.81,;64.1,-18.35,;63.07,-17.2,;61.56,-17.52,;61.08,-18.99,)| Show InChI InChI=1S/C23H32N8/c1-25-23-27-14-20-21(17-4-8-19(9-5-17)30-12-10-26-11-13-30)29-31(22(20)28-23)15-16-2-6-18(24)7-3-16/h4-5,8-9,14,16,18,26H,2-3,6-7,10-13,15,24H2,1H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill
US Patent
| Assay Description
The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... |
US Patent US9744172 (2017)
BindingDB Entry DOI: 10.7270/Q2PR7Z30 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data