

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50384602 CHEMBL2036795::US9744172, Compound UNC00000344A
SMILES: CCCCNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccccc1
InChI Key: InChIKey=JHLDSSUQVZCRDO-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50384602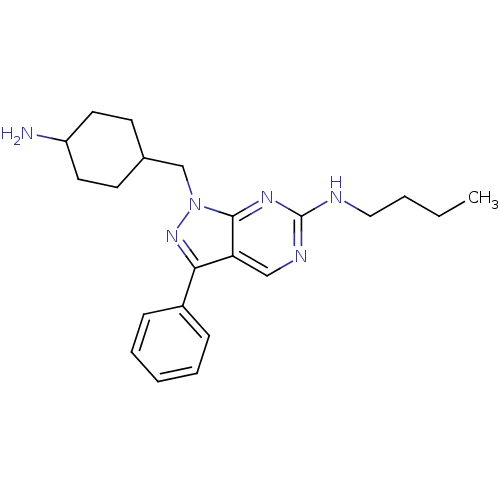 (CHEMBL2036795 | US9744172, Compound UNC00000344A) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Axl using KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assay | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384602 (CHEMBL2036795 | US9744172, Compound UNC00000344A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9744172 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384602 (CHEMBL2036795 | US9744172, Compound UNC00000344A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50384602 (CHEMBL2036795 | US9744172, Compound UNC00000344A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Tyro3 using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assay | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||