

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCOC(=O)c1ccccc1NC(=O)Nc1ccc(OS(N)(=O)=O)cc1
InChI Key: InChIKey=BTDCRIRAHNKHEP-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50387154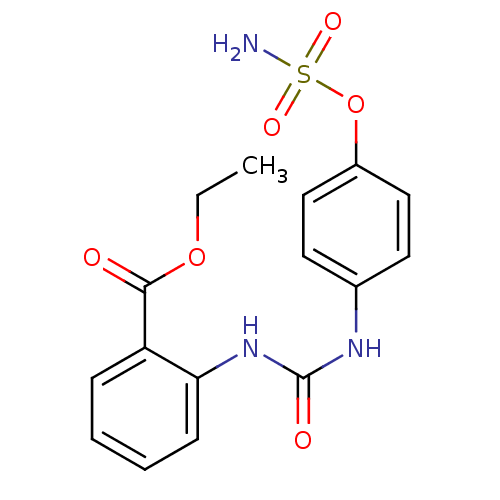 (4-ureidophenyl sulfamate ring derivative 3ar | CHE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Romanian Academy Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 | Bioorg Med Chem 21: 1404-9 (2013) Article DOI: 10.1016/j.bmc.2012.11.004 BindingDB Entry DOI: 10.7270/Q2XW4M4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50387154 (4-ureidophenyl sulfamate ring derivative 3ar | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Romanian Academy Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 | Bioorg Med Chem 21: 1404-9 (2013) Article DOI: 10.1016/j.bmc.2012.11.004 BindingDB Entry DOI: 10.7270/Q2XW4M4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50387154 (4-ureidophenyl sulfamate ring derivative 3ar | CHE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Romanian Academy Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-9 | Bioorg Med Chem 21: 1404-9 (2013) Article DOI: 10.1016/j.bmc.2012.11.004 BindingDB Entry DOI: 10.7270/Q2XW4M4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387154 (4-ureidophenyl sulfamate ring derivative 3ar | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Romanian Academy Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-12 | Bioorg Med Chem 21: 1404-9 (2013) Article DOI: 10.1016/j.bmc.2012.11.004 BindingDB Entry DOI: 10.7270/Q2XW4M4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50387154 (4-ureidophenyl sulfamate ring derivative 3ar | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay | Bioorg Med Chem Lett 22: 4681-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.083 BindingDB Entry DOI: 10.7270/Q20866C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50387154 (4-ureidophenyl sulfamate ring derivative 3ar | CHE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay | Bioorg Med Chem Lett 22: 4681-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.083 BindingDB Entry DOI: 10.7270/Q20866C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50387154 (4-ureidophenyl sulfamate ring derivative 3ar | CHE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology | J Enzyme Inhib Med Chem 29: 571-81 (2014) Article DOI: 10.3109/14756366.2013.827677 BindingDB Entry DOI: 10.7270/Q2NC5ZCQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50387154 (4-ureidophenyl sulfamate ring derivative 3ar | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay | Bioorg Med Chem Lett 22: 4681-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.083 BindingDB Entry DOI: 10.7270/Q20866C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50387154 (4-ureidophenyl sulfamate ring derivative 3ar | CHE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 1 preincubated for 15 mins measured for 10 to 100 sec using phenol red indicator-based stopped flow assay | Bioorg Med Chem Lett 22: 4681-5 (2012) Article DOI: 10.1016/j.bmcl.2012.05.083 BindingDB Entry DOI: 10.7270/Q20866C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||