

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50388338 CHEMBL2059508
SMILES: CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O
InChI Key: InChIKey=CUVFYDOJFCWZIF-GYEBWSHQSA-J
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heparanase (Homo sapiens (Human)) | BDBM50388338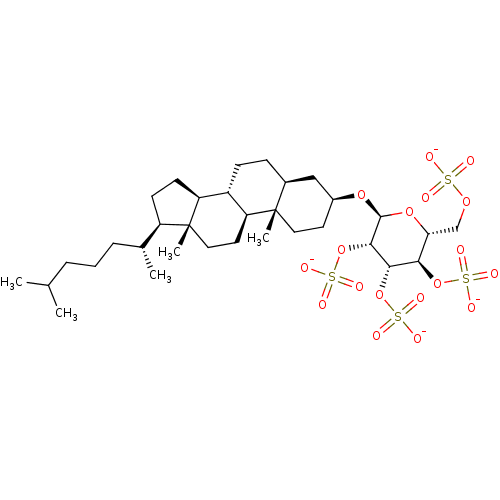 (CHEMBL2059508) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant heparanase after 2 to 24 hrs by WST1 dye based fondaparinux assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic fibroblast growth factor (Homo sapiens (Human)) | BDBM50388338 (CHEMBL2059508) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Binding affinity to FGF-1 by surface plasmon resonance assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50388338 (CHEMBL2059508) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited Curated by ChEMBL | Assay Description Binding affinity to FGF-2 by surface plasmon resonance assay | J Med Chem 55: 3804-13 (2012) Article DOI: 10.1021/jm201708h BindingDB Entry DOI: 10.7270/Q2G161WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||