 Found 6 hits for monomerid = 50388398
Found 6 hits for monomerid = 50388398 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50388398
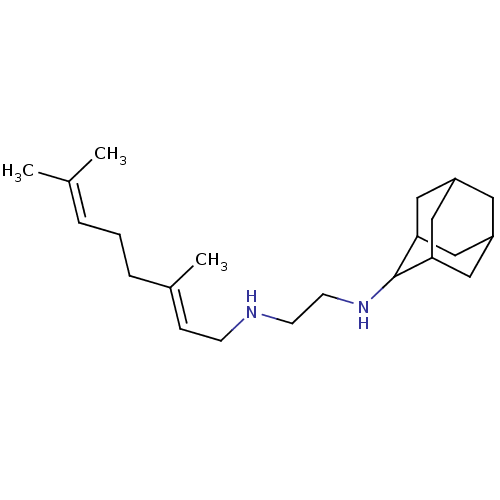
(CHEMBL561057 | SQ-109)Show SMILES CC(C)=CCC\C(C)=C\CNCCNC1C2CC3CC(C2)CC1C3 |TLB:18:19:16.17.23:14,23:22:20:16.17.18,13:14:20:16.17.18,13:14:16.17.23:20.19.21,THB:18:17:14:20.19.21,23:17:20:22.14.21,21:19:16:23.22.14,21:22:16:20.18.19,13:14:16:20.18.19,(-5.14,2.27,;-5.13,1.44,;-5.84,1.02,;-4.41,1.04,;-3.7,1.46,;-2.98,1.06,;-2.28,1.48,;-2.29,2.3,;-1.56,1.08,;-.85,1.5,;-.13,1.09,;.58,1.51,;1.3,1.11,;2.04,1.48,;2.72,1.02,;2.74,.22,;2.11,-.47,;2.91,-.24,;3.67,-.52,;4.2,.17,;3.46,-.03,;4.19,.99,;3.44,1.28,;2.89,.61,)| Show InChI InChI=1S/C22H38N2/c1-16(2)5-4-6-17(3)7-8-23-9-10-24-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-24H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human geranylgeranyl diphosphate synthase |
J Med Chem 57: 3126-39 (2014)
Article DOI: 10.1021/jm500131s
BindingDB Entry DOI: 10.7270/Q27P90ZN |
More data for this
Ligand-Target Pair | |
4,4'-diapophytoene synthase
(Staphylococcus aureus) | BDBM50388398

(CHEMBL561057 | SQ-109)Show SMILES CC(C)=CCC\C(C)=C\CNCCNC1C2CC3CC(C2)CC1C3 |TLB:18:19:16.17.23:14,23:22:20:16.17.18,13:14:20:16.17.18,13:14:16.17.23:20.19.21,THB:18:17:14:20.19.21,23:17:20:22.14.21,21:19:16:23.22.14,21:22:16:20.18.19,13:14:16:20.18.19,(-5.14,2.27,;-5.13,1.44,;-5.84,1.02,;-4.41,1.04,;-3.7,1.46,;-2.98,1.06,;-2.28,1.48,;-2.29,2.3,;-1.56,1.08,;-.85,1.5,;-.13,1.09,;.58,1.51,;1.3,1.11,;2.04,1.48,;2.72,1.02,;2.74,.22,;2.11,-.47,;2.91,-.24,;3.67,-.52,;4.2,.17,;3.46,-.03,;4.19,.99,;3.44,1.28,;2.89,.61,)| Show InChI InChI=1S/C22H38N2/c1-16(2)5-4-6-17(3)7-8-23-9-10-24-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-24H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus CrtM |
J Med Chem 57: 3126-39 (2014)
Article DOI: 10.1021/jm500131s
BindingDB Entry DOI: 10.7270/Q27P90ZN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50388398

(CHEMBL561057 | SQ-109)Show SMILES CC(C)=CCC\C(C)=C\CNCCNC1C2CC3CC(C2)CC1C3 |TLB:18:19:16.17.23:14,23:22:20:16.17.18,13:14:20:16.17.18,13:14:16.17.23:20.19.21,THB:18:17:14:20.19.21,23:17:20:22.14.21,21:19:16:23.22.14,21:22:16:20.18.19,13:14:16:20.18.19,(-5.14,2.27,;-5.13,1.44,;-5.84,1.02,;-4.41,1.04,;-3.7,1.46,;-2.98,1.06,;-2.28,1.48,;-2.29,2.3,;-1.56,1.08,;-.85,1.5,;-.13,1.09,;.58,1.51,;1.3,1.11,;2.04,1.48,;2.72,1.02,;2.74,.22,;2.11,-.47,;2.91,-.24,;3.67,-.52,;4.2,.17,;3.46,-.03,;4.19,.99,;3.44,1.28,;2.89,.61,)| Show InChI InChI=1S/C22H38N2/c1-16(2)5-4-6-17(3)7-8-23-9-10-24-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-24H,4,6,8-15H2,1-3H3/b17-7+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114346
BindingDB Entry DOI: 10.7270/Q2PZ5DT3 |
More data for this
Ligand-Target Pair | |
4,4'-diapophytoene synthase
(Staphylococcus aureus) | BDBM50388398

(CHEMBL561057 | SQ-109)Show SMILES CC(C)=CCC\C(C)=C\CNCCNC1C2CC3CC(C2)CC1C3 |TLB:18:19:16.17.23:14,23:22:20:16.17.18,13:14:20:16.17.18,13:14:16.17.23:20.19.21,THB:18:17:14:20.19.21,23:17:20:22.14.21,21:19:16:23.22.14,21:22:16:20.18.19,13:14:16:20.18.19,(-5.14,2.27,;-5.13,1.44,;-5.84,1.02,;-4.41,1.04,;-3.7,1.46,;-2.98,1.06,;-2.28,1.48,;-2.29,2.3,;-1.56,1.08,;-.85,1.5,;-.13,1.09,;.58,1.51,;1.3,1.11,;2.04,1.48,;2.72,1.02,;2.74,.22,;2.11,-.47,;2.91,-.24,;3.67,-.52,;4.2,.17,;3.46,-.03,;4.19,.99,;3.44,1.28,;2.89,.61,)| Show InChI InChI=1S/C22H38N2/c1-16(2)5-4-6-17(3)7-8-23-9-10-24-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-24H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus ATCC 27659 dehydrosqualene synthase expressed in Escherichia coli BL21(DE3) after 30 mins by spectrophotometric a... |
J Med Chem 55: 4367-72 (2012)
Article DOI: 10.1021/jm300208p
BindingDB Entry DOI: 10.7270/Q2Z320QH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Squalene synthase
(Homo sapiens (Human)) | BDBM50388398

(CHEMBL561057 | SQ-109)Show SMILES CC(C)=CCC\C(C)=C\CNCCNC1C2CC3CC(C2)CC1C3 |TLB:18:19:16.17.23:14,23:22:20:16.17.18,13:14:20:16.17.18,13:14:16.17.23:20.19.21,THB:18:17:14:20.19.21,23:17:20:22.14.21,21:19:16:23.22.14,21:22:16:20.18.19,13:14:16:20.18.19,(-5.14,2.27,;-5.13,1.44,;-5.84,1.02,;-4.41,1.04,;-3.7,1.46,;-2.98,1.06,;-2.28,1.48,;-2.29,2.3,;-1.56,1.08,;-.85,1.5,;-.13,1.09,;.58,1.51,;1.3,1.11,;2.04,1.48,;2.72,1.02,;2.74,.22,;2.11,-.47,;2.91,-.24,;3.67,-.52,;4.2,.17,;3.46,-.03,;4.19,.99,;3.44,1.28,;2.89,.61,)| Show InChI InChI=1S/C22H38N2/c1-16(2)5-4-6-17(3)7-8-23-9-10-24-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-24H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human squalene synthase |
J Med Chem 55: 4367-72 (2012)
Article DOI: 10.1021/jm300208p
BindingDB Entry DOI: 10.7270/Q2Z320QH |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50388398

(CHEMBL561057 | SQ-109)Show SMILES CC(C)=CCC\C(C)=C\CNCCNC1C2CC3CC(C2)CC1C3 |TLB:18:19:16.17.23:14,23:22:20:16.17.18,13:14:20:16.17.18,13:14:16.17.23:20.19.21,THB:18:17:14:20.19.21,23:17:20:22.14.21,21:19:16:23.22.14,21:22:16:20.18.19,13:14:16:20.18.19,(-5.14,2.27,;-5.13,1.44,;-5.84,1.02,;-4.41,1.04,;-3.7,1.46,;-2.98,1.06,;-2.28,1.48,;-2.29,2.3,;-1.56,1.08,;-.85,1.5,;-.13,1.09,;.58,1.51,;1.3,1.11,;2.04,1.48,;2.72,1.02,;2.74,.22,;2.11,-.47,;2.91,-.24,;3.67,-.52,;4.2,.17,;3.46,-.03,;4.19,.99,;3.44,1.28,;2.89,.61,)| Show InChI InChI=1S/C22H38N2/c1-16(2)5-4-6-17(3)7-8-23-9-10-24-22-20-12-18-11-19(14-20)15-21(22)13-18/h5,7,18-24H,4,6,8-15H2,1-3H3/b17-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human SQS |
J Med Chem 57: 3126-39 (2014)
Article DOI: 10.1021/jm500131s
BindingDB Entry DOI: 10.7270/Q27P90ZN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data