

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50388926 CHEMBL2063395
SMILES: CCC[C@@H]1NC(=O)[C@H](C)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O
InChI Key: InChIKey=LHUGWXWDPVKZOS-DBXVSBKESA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50388926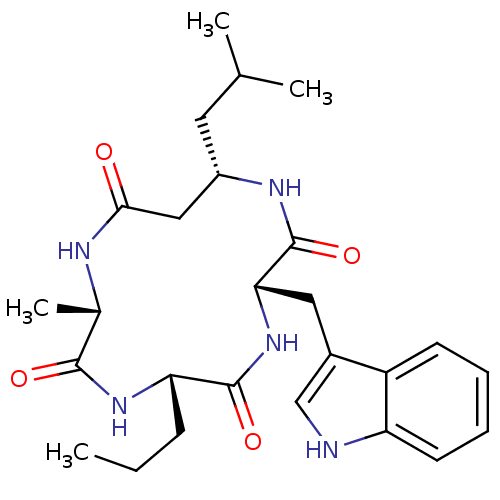 (CHEMBL2063395) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Competitive inhibition of HDAC1 using Ac-Lys(Ac)-AMC as substrate by Lineweaver-Burk plot analysis | ACS Med Chem Lett 3: 505-508 (2012) Article DOI: 10.1021/ml300081u BindingDB Entry DOI: 10.7270/Q2JQ122X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50388926 (CHEMBL2063395) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 using Ac-Lys(Ac)-AMC as substrate after 30 mins by fluorescence analysis | ACS Med Chem Lett 3: 505-508 (2012) Article DOI: 10.1021/ml300081u BindingDB Entry DOI: 10.7270/Q2JQ122X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50388926 (CHEMBL2063395) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC2 using Ac-Lys(Ac)-AMC as substrate after 30 mins by fluorescence analysis | ACS Med Chem Lett 3: 505-508 (2012) Article DOI: 10.1021/ml300081u BindingDB Entry DOI: 10.7270/Q2JQ122X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50388926 (CHEMBL2063395) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Cell and Molecular Biology, The Scripps Research Institute, La Jolla, CA, USA; National Yang-Ming University, Taipei, Taiwan; National Yang-Ming University Hospital, Ilan, Taiwan. Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) | Bioorg Med Chem Lett 27: 3289-3293 (2017) Article DOI: 10.1016/j.bmcl.2017.06.027 BindingDB Entry DOI: 10.7270/Q2Z32238 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50388926 (CHEMBL2063395) | PDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Cell and Molecular Biology, The Scripps Research Institute, La Jolla, CA, USA; National Yang-Ming University, Taipei, Taiwan; National Yang-Ming University Hospital, Ilan, Taiwan. Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) | Bioorg Med Chem Lett 27: 3289-3293 (2017) Article DOI: 10.1016/j.bmcl.2017.06.027 BindingDB Entry DOI: 10.7270/Q2Z32238 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50388926 (CHEMBL2063395) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Cell and Molecular Biology, The Scripps Research Institute, La Jolla, CA, USA; National Yang-Ming University, Taipei, Taiwan; National Yang-Ming University Hospital, Ilan, Taiwan. Curated by ChEMBL | Assay Description Inhibition of HDAC1 (unknown origin) | Bioorg Med Chem Lett 27: 3289-3293 (2017) Article DOI: 10.1016/j.bmcl.2017.06.027 BindingDB Entry DOI: 10.7270/Q2Z32238 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2) (Homo sapiens (Human)) | BDBM50388926 (CHEMBL2063395) | PDB KEGG GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC3/NCoR2 using Ac-Lys(Ac)-AMC as substrate after 30 mins by fluorescence analysis | ACS Med Chem Lett 3: 505-508 (2012) Article DOI: 10.1021/ml300081u BindingDB Entry DOI: 10.7270/Q2JQ122X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50388926 (CHEMBL2063395) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 using Ac-Lys(Ac)-AMC as substrate after 30 mins by fluorescence analysis | ACS Med Chem Lett 3: 505-508 (2012) Article DOI: 10.1021/ml300081u BindingDB Entry DOI: 10.7270/Q2JQ122X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||