

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCOc1ccc2cc(ccc2n1)-c1nn(CC2CCNCC2)c2ncnc(N)c12
InChI Key: InChIKey=ZOVKFJPIFCBGJO-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50389684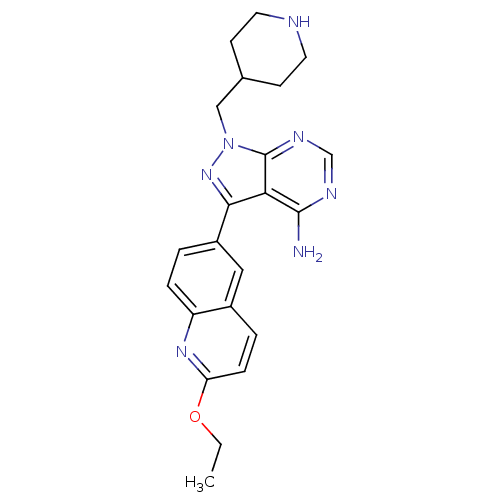 (CHEMBL2070051 | US10307425, Example 119 | US106321...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human SRC using Ac-EIYGEFKKK-OH as substrate after 60 mins by phosphorimaging method | J Med Chem 55: 2416-26 (2012) Article DOI: 10.1021/jm201713h BindingDB Entry DOI: 10.7270/Q2P2706V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50389684 (CHEMBL2070051 | US10307425, Example 119 | US106321...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human ABL using EAIYAAPFAKKK-OH as substrate by phosphorimaging method | J Med Chem 55: 2416-26 (2012) Article DOI: 10.1021/jm201713h BindingDB Entry DOI: 10.7270/Q2P2706V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50389684 (CHEMBL2070051 | US10307425, Example 119 | US106321...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human LCK using Ac-EIYGEFKKK-OH as substrate after 60 mins | J Med Chem 55: 2416-26 (2012) Article DOI: 10.1021/jm201713h BindingDB Entry DOI: 10.7270/Q2P2706V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 (Homo sapiens (Human)) | BDBM50389684 (CHEMBL2070051 | US10307425, Example 119 | US106321...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human EPHA3 using myelin basic protein as substrate after 120 mins | J Med Chem 55: 2416-26 (2012) Article DOI: 10.1021/jm201713h BindingDB Entry DOI: 10.7270/Q2P2706V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calmodulin-domain protein kinase 1 (Toxoplasma gondii) | BDBM50389684 (CHEMBL2070051 | US10307425, Example 119 | US106321...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington US Patent | Assay Description Inhibitors were evaluated in triplicate in eight-point dilutions (3-fold dilutions) during the enzymatic reactions. TgCDPK1 enzymatic inhibition was ... | US Patent US10632122 (2020) BindingDB Entry DOI: 10.7270/Q2M048G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50389684 (CHEMBL2070051 | US10307425, Example 119 | US106321...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human EGFR using poly glu-Tyr as substrate after 30 mins | J Med Chem 55: 2416-26 (2012) Article DOI: 10.1021/jm201713h BindingDB Entry DOI: 10.7270/Q2P2706V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50389684 (CHEMBL2070051 | US10307425, Example 119 | US106321...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human p38alpha using myelin basic protein as substrate after 180 mins | J Med Chem 55: 2416-26 (2012) Article DOI: 10.1021/jm201713h BindingDB Entry DOI: 10.7270/Q2P2706V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CAM kinase, CDPK family TgCDPK1 (Toxoplasma gondii (strain ATCC 50861 / VEG)) | BDBM50389684 (CHEMBL2070051 | US10307425, Example 119 | US106321...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington US Patent | Assay Description Inhibitors were evaluated in triplicate in eight-point dilutions (3-fold dilutions) during the enzymatic reactions. TgCDPK1 enzymatic inhibition was ... | US Patent US10307425 (2019) BindingDB Entry DOI: 10.7270/Q2862JT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM50389684 (CHEMBL2070051 | US10307425, Example 119 | US106321...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human CSK using Ac-KKKKEEIYFFF-OH as substrate after 180 mins | J Med Chem 55: 2416-26 (2012) Article DOI: 10.1021/jm201713h BindingDB Entry DOI: 10.7270/Q2P2706V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||