

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50393748 CHEMBL2159207
SMILES: CC(=O)N[C@H]1CC[C@@H](CC1)n1c(C)nc2cnc3[nH]ccc3c12
InChI Key: InChIKey=KWKMUTBWQNMORT-JOCQHMNTSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50393748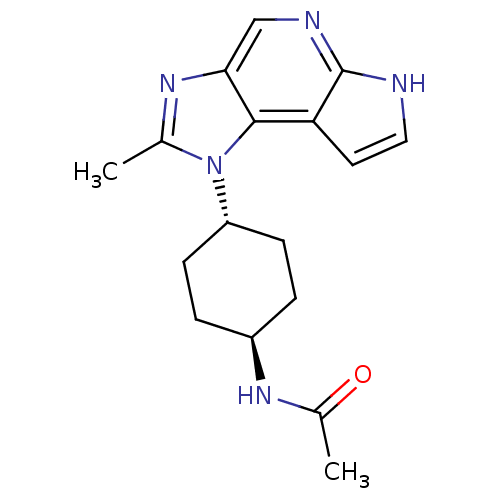 (CHEMBL2159207) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of JAK1 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... | J Med Chem 55: 6176-93 (2012) Article DOI: 10.1021/jm300628c BindingDB Entry DOI: 10.7270/Q25Q4X6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50393748 (CHEMBL2159207) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of JAK2 kinase domain using N-terminal 5-carboxyfluorescein-tagged Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 m... | J Med Chem 55: 6176-93 (2012) Article DOI: 10.1021/jm300628c BindingDB Entry DOI: 10.7270/Q25Q4X6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50393748 (CHEMBL2159207) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of JAK2 in human TF1 cells assessed as inhibition of EPO-induced STAT5 phosphorylation incubated for 20 mins prior to EPO-induction measur... | J Med Chem 55: 6176-93 (2012) Article DOI: 10.1021/jm300628c BindingDB Entry DOI: 10.7270/Q25Q4X6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50393748 (CHEMBL2159207) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of JAK1 in human TF1 cells assessed as inhibition of IL6-induced STAT3 phosphorylation incubated for 20 mins prior to IL6-induction measur... | J Med Chem 55: 6176-93 (2012) Article DOI: 10.1021/jm300628c BindingDB Entry DOI: 10.7270/Q25Q4X6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||